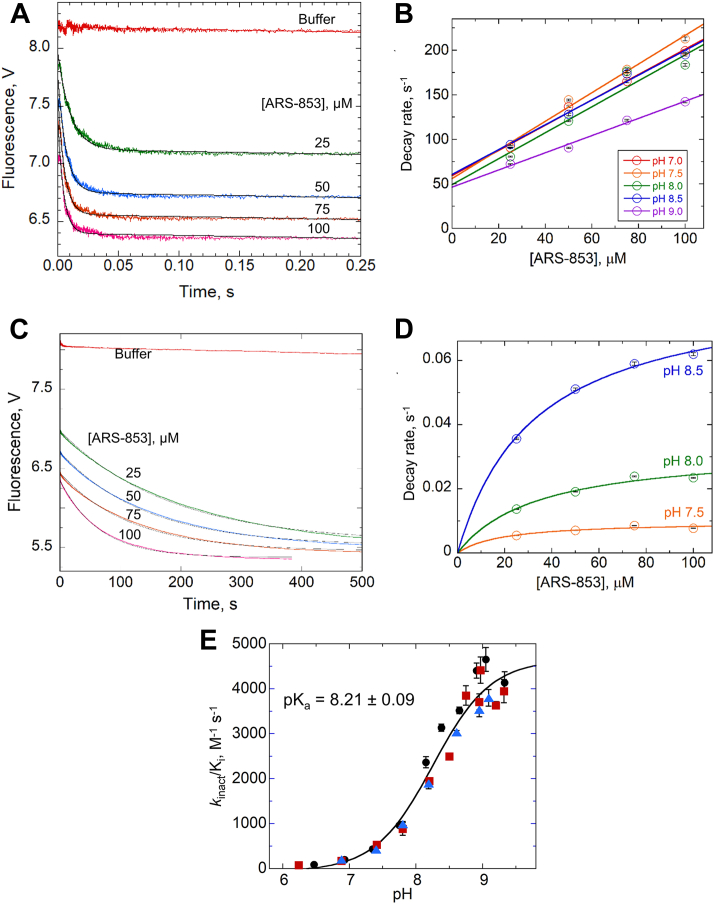
Figure 3.
Stopped-flow kinetic studies of KRASCCLWfluorescence changes upon ARS-853 addition show a very fast fluorescence decrease followed by a slower decrease, and a functional pKaof ∼8.2 for the slow step.A, stopped-flow kinetic data of 5 μM KRASCCLW (G12C,”Cys-light”, Y137W) mixed with buffer or ARS-853 at 25, 50, 75, and 100 μM at 5 °C and pH 7.5 demonstrate an initial fast drop in fluorescence (<50 ms). Data shown are representative of three or more independent experiments. B, plots of the pseudo first order rate constant versus ARS-853 concentration at five pH values (average plus or minus the standard error of each) yield a linear fit and an absence of a pH effect up to pH 8.5. C, a further decrease in fluorescence beyond that seen in (A) occurs over ∼500 s at 5 °C (same colors and concentrations as in (A). Data shown are representative of three or more independent experiments. D, pseudo-first order rate constants at 5 °C fit to a hyperbola are consistent with a saturable step (interpreted as covalent modification). As shown, rate constants are strongly affected by pH. E, second-order rate constants (kinact/Ki) for the slow step of KRASCCLW and ARS-853 reactions were fit to a Boltzmann sigmoidal; the ARS-853 functional pKa was found to be 8.21 ± 0.09 at 20 °C. Three colors and marker shapes represent three independent trials, and each data point reports the mean ± SEM for the kinact/Ki value derived from multiple [ARS-853]; all data were used in the final fit.