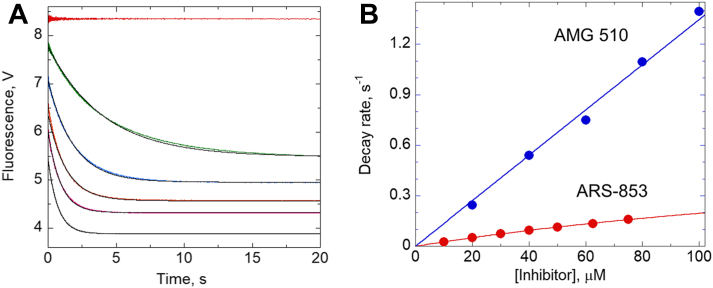
Figure 4.
Kinetic studies of KRASCCLWfluorescence changes with increasing inhibitor concentrations (at 20 °C) are faster for AMG 510 than for ARS-853.A, stopped-flow kinetic data of 5 μM KRASCCLW mixed with buffer or AMG 510 at 20, 40, 60, 80, and 100 μM at 20 °C and pH 8.6 (faster fluorescence decreases reflect higher concentrations of inhibitor) demonstrate faster kinetics for AMG 510 reactions than for “slow step” reactions with ARS-853. B, plots of the observed rates versus inhibitor concentrations suggest very high Ki values for both inhibitors (Ki > 100 μM) under these conditions. Fitted values for kinact/Ki are 2770 ± 10 and 14,000 ± 100 M−1 s−1 with ARS-853 and AMG 510, respectively.