FIGURE 8.
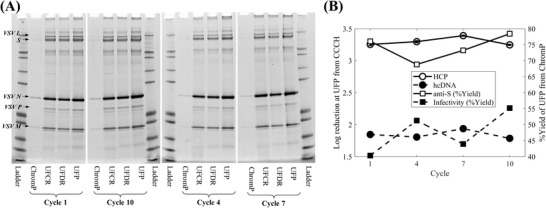
Evaluation of the impact of cleaning in place strategy on the purity and yield of the final purified product (UFP) obtained by processing the chromatography flowthrough product pool (ChromP) through ultrafiltration/diafiltration for lab‐scale resin re‐use cycles 1, 4, 7 and 10: (A) SDS‐PAGE analysis of clarified cell culture harvest (CCCH), ChromP, concentrated ChromP (UFCR), diafiltered ChromP (UFDR) and UFP; (B) In left‐hand side y‐axis, log reduction of host cell protein (HCP) (○) and DNA (hcDNA) (●) impurities at UFP from CCCH obtained by ELISA and qPCR analysis, respectively. On the right‐hand side y‐axis, step yield at UFP from ChromP based on anti‐Spike (S) protein quantitative western blotting (□) and infectivity data (■). In (B), the HCP log reduction data error bars correspond to ± 1 standard deviation (sd) from analytical replicates
