Abstract
Cortisol and corticosterone, hormones traditionally considered biomarkers of stress, can be measured in fluid biomatrices (e.g., blood, saliva) from live animals to evaluate conditions at sampling time, or in solid biomatrices (e.g., hair, feather) from live or dead animals to obtain information regarding long-term changes. Using these biomarkers to evaluate physiological stress responses in domestic animals may be challenging due to the diverse characteristics of biomatrices for potential measurement. Ideally, a single measurement from the biomatrix should be sufficient for evaluating chronic stress. The availability of appropriate and cost-effective immunoassay methods for detecting the biomarkers should also be considered. This review discusses the strengths and limitations of different biomatrices with regard to ensuring the highest possible reliability for chronic stress evaluation. Overall, solid biomatrices require less frequent sampling than other biomatrices, resulting in greater time- and cost-effectiveness, greater ease of use, and fewer errors. The multiplex immunoassay can be used to analyze interactions and correlations between cortisol and other stress biomarkers in the same biomatrix. In light of the lack of information regarding appropriate biomatrices for measuring chronic stress, this review may help investigators set experimental conditions or design biological research.
Keywords: Animals, Stress biomarkers, Biomatrix, Enzyme immunoassay, Stress
INTRODUCTION
The hormones cortisol and corticosterone have become increasingly important in animal research as key biological markers (biomarkers) of stress and distress. Selection and preparation of the appropriate biological matrix (biomatrix) is the first step in every analytical procedure in biological research and is especially important for evaluating response to chronic stress. Biomatrices can be divided into three types: fluid (e.g., blood, saliva, urine, milk), semi-solid (e.g., earwax, bone marrow, feces), and solid (e.g., hair, wool, feathers, nails, scales). Each of the aforementioned types has advantages and disadvantages, with its own specific properties. The aims of this review are the following: (1) to discuss the biomarkers of stress in different animal species; (2) to provide guidelines for selecting the appropriate biomatrix for studying chronic stress in animals according to composition, consistency, and preparation technique; and (3) to discuss the strengths and limitations of various laboratory techniques for analyzing stress hormone levels. In particular, the review considers the questions of which biomatrices most accurately represent stress levels and whether stress in animals can be evaluated accurately using only a single biomarker.
BIOMARKERS OF STRESS IN ANIMALS
Biomarkers are defined as biological molecules whose levels in various biomatrices are used to evaluate whether an organism’s condition is normal or abnormal [1]. When evaluating response to a particular stress, it is critical to identify the most reliable biomarker; its levels should be strongly correlated with the specific pathophysiological aspects of that stress [2]. In animals, stress causes the endocrine system [3] to release several hormones, such as epinephrine, norepinephrine, and cortisol [2,4,5]. In general, researchers selecting biomarkers should consider the following: (1) the ease of collecting and processing the required biological specimens; (2) the stability and durability of the marker throughout the storage and evaluation periods; and (3) the availability of assays with sufficient specificity and sensitivity to the markers selected [2]. Cortisol and corticosterone score well on these criteria and are considered the classical biomarkers of stress, used more widely in animal research than other biomarkers such as prolactin, serum amyloid A, and substance P.
Cortisol
In general, the term “stress” refers to a normal reaction of an animal’s body to any type of physiological change (e.g., fluctuations in heart or respiration rate), behavioral change (e.g., decreasing feed intake, changes in time spent standing or lying down), or stressor (e.g., changes in environment or nutrition) [6,7]. Once the animal’s central nervous system (CNS) processes the stress, four biological responses are triggered: (1) the autonomic nervous system response, (2) the neuroendocrine system response, (3) the immune system response, and (4) the behavioral response [8]. In this regard, a series of hormones are synthesized and then released into the bloodstream, influencing the sympathetic–adrenal–medullary axis, the hypothalamic–pituitary–adrenal (HPA) axis, inflammation, metabolism, and immune function [6,9]. The HPA axis is the primary system involved in the initiation and coordination of the stress response [6]. Activation of the HPA axis stimulates secretion of corticotropin-releasing hormone from the paraventricular nucleus, which then stimulates the anterior pituitary to release adrenocorticotropic hormone (ACTH) [6,10]. ACTH then stimulates the adrenal cortex to secrete cortisol, which is the principal glucocorticoid hormone in humans, non-human primates, and other large mammals [6,10]. In addition, the regulatory role of central HPA axis on the production of cortisol can be influenced by the peripheral HPA axis stimulation such as wound or dryness of skin [11,12]. Under normal body conditions, blood levels of both free and bound cortisol are subject to circadian rhythms, being highest in the early morning and decreasing throughout the day [13]. As cortisol is a biomarker of HPA axis activity and affects blood glucose levels, blood pressure, and other parameters, abnormally high cortisol is considered an indicator of physiological stress (acute or chronic). Many previous studies in different animal species have used cortisol as a biomarker of stress (Table 1).
Table 1. Use of cortisol as a biomarker in previous studies of stress in animals.
| Stress type | Species | Biomatrices |
|---|---|---|
| Acute | Cow | Blood [14], saliva [14,15], urine [16], milk [17], feces [18] |
| Sheep | Blood [19], saliva [19], urine [20], feces [19] | |
| Pig | Blood [21], saliva [22,23], urine [24], feces [25] | |
| Fish | Blood [26], respiratory vapor [27] | |
| Chronic | Cow | Hair [28], hooves and claws [29] |
| Sheep | Wool [30] | |
| Pig | Hair [31] | |
| Fish | Scales [32], fins [33] |
Corticosterone
Corticosterone is the main glucocorticoid hormone in birds and rodents, among others. Like cortisol, it is secreted by the adrenal cortex in response to stimulation by ACTH. Activation of the HPA axis in response to stressors results in increased blood corticosterone concentration, usually 1–2 min after exposure. Approximately 90%–95% of cortisol and corticosterone in blood is bound to protein (globulin or albumin) [34]; only the free steroids have ready access to the corticosteroid receptors in the target cells. Table 2 summarizes previous studies that measured corticosterone in different biomatrices as a biomarker of stress in different species. Measuring corticosterone levels can be useful for evaluating animal behavior and welfare, especially that of birds. For instance, rearing commercial broiler chickens under stressful conditions (e.g., high stock density) causes the secretion of corticosterone into the blood as an adaptive response [34,35], which can negatively impact their immune system, making them more prone to health risks such as severe infections; this can in turn cause health issues for consumers.
Table 2. Use of corticosterone as a biomarker in previous studies of stress in animals.
Other biomarkers of stress
Multiple blood biomarkers respond to stress in different situations. Cortisol and corticosterone are the main stress hormones used to assess the level of stress in animals. However, according to some studies [42,43], prolactin can also be used as a biomarker of acute and chronic stress in fish, cattle, sheep, donkeys, rats, dogs, and humans. It is released from the pituitary gland in response to eating, mating, and other stimuli. In mammals, its main function is to stimulate the mammary gland to produce milk, and it also plays active roles in reproduction, homeostasis, and the immune response [42]. Serum levels of the protein amyloid A are elevated under stress, for instance in cattle [44,45], and can be used to study stress levels in early life. Other biomarkers that can be used as reliable stress indicators include lactate, glucose, and substance P [45].
STRESS AND COMMON STRESSORS
Acute and chronic stress responses
Both humans and animals may be subjected to acute or chronic stress by exposure to stressors. Stressors may be physical (e.g., fluctuations in air temperature and relative humidity, fluctuations in food and water availability, low blood sugar, injury, hemorrhage, transportation, painful procedures such as castration) or psychological (weaning, restraint, separation from peers and introduction to unknown animals, reproductive competition, nursing offspring) [10,34,46,47]. Thus, many different stressors may trigger acute or chronic stress reactions.
Acute stress reactions normally occur in response to a brief threat and are characterized by quick recovery of physiological balance, leading to complete adaptation [48–50]. During an acute stress response, the HPA axis is activated and hormones are secreted over a few seconds or minutes, resulting in physiological and metabolic changes such as increased heart rate, faster breathing, increased blood pressure, increased energy mobilization, stimulation of immune function, reduced appetite, reduced plasma potassium and magnesium, reduced digestive flow rate in the rumen and stomach, and increased digestive flow rate in the intestines [48].
In contrast to acute stress, chronic stress is a persistent physiological condition [51] that occurs when the body experiences several stressors or repeated acute stress, preventing the autonomic nervous system from activating normal physiological and behavioral adaptations [3,52]. Therefore, alteration of biological functions and induction of distress are expected during chronic stress. Such long-term over-stimulation of coping responses may result in direct effects, such as increased body heat, low energy, and anxiety, or indirect effects, such as functional changes in the endocrine, immune, and metabolic systems. These effects generally lead to prepathological or pathological consequences that reduce health and welfare [3,53].
Comparison of acute and chronic stress
The two types of stress are distinguished by duration and intensity. Stress lasting for a period of minutes to hours is classified as acute stress, whereas chronic stress persists for several hours per day, for weeks, or for months [54]. Previous animal studies evaluating responses to acute stress, such as castration and weaning, have measured cortisol concentration in various fluid biomatrices, such as plasma, serum, saliva, urine, and milk [34,55–57] (Table 1). However, evaluation of chronic stress (e.g., fluctuations in food and water availability, prolonged high temperature), especially in a single measurement, is a more complex process requiring careful assessment of the characteristics and limitations of the biomatrix from which stress biomarkers are to be extracted [58]. Measuring stress biomarkers in fluid biomatrices can only provide information about a single moment in time; however, this problem can be overcome by studying solid biomatrices such as hair, feathers, nails, and scales, which can provide information about changes in cortisol and corticosterone concentrations over time.
Common stressors
The main stressors of animals can be classified as environmental (e.g., toxic metal pollution, chemical fertilizer use, pesticide contamination), physiological (e.g., heat stress, cold stress, dehydration, advanced pregnancy), nutritional (e.g., acidosis, ketosis, hypocalcemia, hypomagnesemia, mycotoxins, and plant toxins), or management-related (handling and transportation, seasonal changes) [59]. Recently, much attention has been focused on heat and cold stress related to climate change, as climate change is a stressor common to all animals worldwide, as opposed to more localized nutritional and environmental factors. Researchers have begun to explore the influence of climatic and meteorological factors on the health and welfare of both animals and humans, particularly in the context of warming due to climate change. In commercial animal farming, environmental factors such as radiation, air movement, precipitation, air temperature, and relative humidity should all be considered to minimize the risk of heat stress [60–62]. Heat stress occurs when an animal’s heat load is higher than its ability to dissipate heat, damaging its general health; immunity, productivity, and welfare can be severely affected [62–64]. Heat stress triggers the release of stress hormones such as cortisol and epinephrine, which cause the body core temperature to rise further, weakening the immune system.
BIOMATRICES
To ensure good study design, the biomatrix needs to be selected and prepared carefully, as each type of biomatrix has its own properties that may complicate the study. In general, animal biomatrices can be classified as (1) fluid (blood, including serum and plasma; urine; saliva; sweat; milk), (2) semi-solid (earwax, bone marrow, feces), or (3) dry and solid (hair, feathers, wool, scales, nails, claws, hooves, teeth, and bone). Studies have shown that cortisol and corticosterone can be measured in all of the aforementioned biomatrices. To ensure the highest reliability and accuracy, biomatrix selection for a study should be based on physicochemical properties, biomatrix type (fluid, semi-solid, or solid), ease of collection, and method of analysis.
Fluid biomatrices
Cortisol and corticosterone can be measured in all types of fluid biomatrices because their chemical properties permit them to pass through all membranes of the body without hindrance [65]. It is normal for blood levels of these biomarkers to fluctuate throughout the day, and the time of sampling (morning or afternoon) should be taken into account when interpreting the results. Table 3 compares the properties of various fluid biomatrices commonly used for analysis of stress biomarkers in animals.
Table 3. Properties of fluid biomatrices with regard to measurement of cortisol and corticosterone.
| Properties | Fluid biomatrix | |||
|---|---|---|---|---|
| Blood | Saliva | Urine | Milk | |
| Invasiveness of sampling | High [57,66], painful | Low [66], painless | Low [66], painless | Low [67], painless |
| Risk of contamination from external sources | Low | High [66] | High | Low |
| Training required for sampling | Yes [57,66] | No | No [66] | No |
| Possibility of repeated sampling | Yes | Yes [68] | Yes | Yes |
| Ethical and legal licenses required | Yes | Yes | Yes | No |
| Biosecurity concerns | Yes | Yes | Yes | Yes |
| Results depend on time of sampling | Yes | Yes [68] | Yes | Yes |
| Results depend on sampling location | Yes | No | No | No |
| Steroid medications may confound results | Yes | Yes [68] | Yes | Yes |
| Sampling requires live animals | Yes | Yes | Yes | Yes |
| Sample preparation is laborious and time consuming | Yes | Yes | Yes | Yes |
| Storage conditions | Refrigerator, freezer [69] | Refrigerator, freezer | Refrigerator, freezer | Refrigerator, freezer [70] |
| Time range represented by biomarker measurement | Single point, hour | Single point, hour | 12–24 h | 8–12 h |
| Biomarker forms represented | Bound, unbound [66] | Unbound [66] | Unbound | Bound, unbound |
| Sampling procedure may confound results | Possibly | Possibly | No | No |
| Stress type | Acute [71] | Acute [71] | Acute [71] | Acute [70] |
| Analytical cost | Relatively expensive | Relatively expensive [72] | Relatively expensive | Relatively expensive |
Blood
Blood is the biomatrix most frequently used for monitoring stress biomarkers (cortisol, corticosterone, and others). Only 5%–10% of blood cortisol is free and biologically active; the rest is bound to proteins such as corticosteroid-binding globulin [73]. When analyzing levels of stress biomarkers in blood, one should consider the amount to be collected, the centrifugation procedure if applicable (whether to separate plasma or serum, which anticoagulant to use), and storage conditions (room temperature, refrigerator, freezer, or otherwise). Generally, blood and other fluid biomatrices should be frozen until analysis. Plasma is the yellow part obtained after centrifuging blood with an anticoagulant (10 min at 1,000×g), whereas serum is obtained by centrifuging blood that has coagulated (10 min at 1,000−2,000×g); both can be stored frozen, preferably at either −20°C or −80°C [74].
Saliva
Recent advances in technology have made it easier to measure cortisol from biological fluids other than blood, such as saliva. Measuring cortisol in saliva has numerous advantages over measuring it in blood. Like blood cortisol, salivary cortisol provides a measure of body cortisol levels within the past few minutes. However, unlike most cortisol in blood, salivary cortisol is free (not bound to other substances). The saliva matrix consists of 99.5% water, and sample collection with saliva-collecting devices is simple and non-invasive. Note that collection from rabid animals is inadvisable. Saliva samples can be stored at −20°C or −80°C [74].
Urine
Urine is another biomatrix commonly used to monitor stress biomarkers. Measuring urinary cortisol or corticosterone provides a measure of the secretion of these substances over 12–24 h. Urine is normally a clear or translucent fluid. It contains approximately 98% water [74], along with sodium, ammonia, phosphate, sulfate, urea, creatinine, proteins, and products processed by the kidney and liver, including drugs and metabolites [75,76]. Urinalysis is used in humans and animals to test for pregnancy, various disorders, and the presence of drugs in the body. The collection of urine from humans and most domestic animals is non-invasive and simple; however, collecting urine from wild animals may be difficult, as they are likely to be less cooperative. Under such circumstances, urine collection bags with catheters may be needed. Urine can be collected in plastic containers of a suitable volume, then stored at −20°C or −80°C [74].
Milk
Milk is a complex biomatrix to analyze due to its high content of nutrients, including proteins, and its variable composition, which may change throughout the nursing period. Mormède et al. [34] reported that cortisol levels in dairy milk are correlated with levels in blood; however, changes in milk composition could potentially affect the analysis results. Milk can only be collected from mature female animals, often only at a certain time of year, and cannot be collected from immature animals.
Semi-solid biomatrices
Generally, instruments for measuring stress biomarkers are designed to measure them in fluid biomatrices. Thus, semi-solid biomatrices may require dilution or dissolution before the measurement can be performed. Biomatrices consisting of soft to hard substances with high viscosity can be classified as semi-solid. Table 4 explains the properties of semi-solid biomatrices that can be used for measurement of stress biomarkers in animals.
Table 4. Properties of semi-solid biomatrices with regard to measurement of cortisol and corticosterone.
| Properties | Semi-solid biomatrix | |
|---|---|---|
| Feces | Earwax | |
| Invasiveness of sampling | Low [77] | Low [77] |
| Risk of contamination from external sources | Yes [78] | No |
| Training required for sampling | No | Yes |
| Sampling procedure may confound results | No | No |
| Possibility of repeated sampling | Yes | No |
| Ethical and legal licenses required | No [77] | Yes [79] |
| Biosecurity concerns | Yes | Yes |
| Results depend on time of sampling | Yes | No |
| Results depend on sampling location | No | No |
| Steroid medications may confound results | Yes | No |
| Sampling requires live animals | Yes | Yes |
| Sample preparation is laborious and time consuming | No | Yes |
| Storage conditions | Refrigerator, freezer | Refrigerator, freezer |
| Time range represented by biomarker measurement | 2–4 days | Week/month |
| Biomarker forms represented | Unbound | Unbound |
| Stress type | Acute | Chronic [77] |
| Analytical cost | Relatively inexpensive | Relatively inexpensive |
Earwax
Recent studies have introduced measurement of cortisol in earwax as a new method for monitoring chronic stress in humans [77] and whales [80]. Earwax collection is non-invasive and pain-free by comparison with the invasive sampling techniques required to collect many commonly used biomatrices [71,79].
Feces
Collection of fecal samples has the advantages of being non-invasive and not requiring ethical approval. As animals drop feces naturally, they can be collected without disturbing the animal [81], making sample collection easier than for other biomatrices. However, fecal samples contain a mixture of many hormones and enzymes at different stages of metabolism, which can interfere with the results. Furthermore, they may contain zoonotic pathogens such as Salmonella, which can increase one’s risk of catching an illness. Analysis of feces from mammals (both ruminant and non-ruminant) and birds is useful for assessing a variety of parameters, including long-term stress, seasonal hormone patterns, and pregnancy status. Feces are typically inhomogeneous, with a composition depending on diet, microbial activity, and the presence of digestive disorders. Feces can easily be collected in suitable bags and stored at −20°C or −80°C [74].
Solid biomatrices
Measurement of stress biomarkers in solid biomatrices, for instance keratinized tissues such as hair and wool, has been validated as a method of monitoring chronic stress in animals such as rhesus macaques and ewes [30,82]. However, there is room to improve the accuracy of this method by further research into factors that influence marker levels in these biomatrices over the long term. Table 5 presents the properties of solid biomatrices that can be used for stress biomarker measurement in animals.
Table 5. Properties of solid biomatrices with regard to measurement of cortisol and corticosterone.
| Properties | Solid biomatrix | |||
|---|---|---|---|---|
| Hair | Feathers | Nails/claws/ hooves | Scales | |
| Invasiveness of sampling | Low [82,83] | Low | Low [71,84] | Low [71,85] |
| Risk of contamination from external sources | Yes | Yes | No | No |
| Training required for sampling procedure | No [57] | No [40] | No | No |
| Sampling procedure may confound results | No | No | No | No |
| Possibility of repeated sampling | Yes | Yes | Yes | No |
| Ethical and legal licenses required | Yes | Yes [40] | No | Yes [85] |
| Biosecurity concerns | Low | Low [86] | Low | Low |
| Results depend on time of sampling | No [87] | No [40] | No | No |
| Results depend on sampling location | Yes [57] | Yes | No | No |
| Steroid medications may confound results | No | No | No | No |
| Sampling requires live animals | No | No [86] | No | No |
| Sample preparation is laborious and time consuming | Yes | Yes | Yes | Yes |
| Storage conditions | Room temperature [87] | Room temperature [40] | Room temperature [84] | Refrigerator, freezer [85] |
| Time range represented by biomarker measurement | Week, month, year | Week, month, year | Week, month, year | Week, month, year |
| Biomarker forms represented | Unbound [88] | Unbound [88] | Unbound [88] | Unbound [88] |
| Stress type | Chronic [82,83] | Chronic [67,86] | Chronic [71,84] | Chronic [71,85] |
| Analytical cost | Relatively inexpensive [72] | Relatively inexpensive [40] | Relatively inexpensive | Relatively expensive |
Hair
Cirimele et al. [89] and Koren et al. [90] introduced methods for measuring cortisol in hair in order to monitor chronic stress response without the result being affected by sampling conditions, such as time of sampling, food intake, background stress levels at sampling time, and stress caused by sampling. Hair cortisol levels reflect blood levels during the period of the hair’s growth, enabling hair to capture information about how cortisol concentrations have changed over time and thus making it a useful biomatrix for monitoring animal welfare and health under exposure to environmental stressors [14].
All biomatrices have their own limitations with regard to sampling. Analysis of hair cortisol concentration is increasingly used to study HPA axis activity in response to prolonged stressful conditions and has proven to be a very informative tool for evaluating chronic stress and welfare in many animal species. However, the results can be influenced by numerous factors, such as genetic inheritance [91,92], species [93,94], physiological stage of development, life history, sex, age [94,95], environmental factors [67], pregnancy [95,96], and parturition [14], as well as methodological factors (storage time and conditions, localization of sample) and color [92]. Hair is a useful biomatrix for retrospective measurements of special analytes (e.g., cortisol) because small amounts of biological molecules present in blood can be deposited in it. Generally, there are two main possible pathways by which cortisol incorporation into hair shaft. One way is thought to be from the vascular system directly to the follicular cells that generate the hair shaft. Another way is through the diffusion of tissues surrounding the follicle of hair [55]. Due to lipophilic properties of cortisol, it is bound to carrier proteins to pass freely across the cellular membranes of target matrices. In humans, head hair grows at up to approximately 1.3 cm per month, whereas hair on other parts of the body grows very slowly. Hair samples can be stored at room temperature until analysis.
Feathers
Like hair, feathers form a keratinized biomatrix. Previous studies have validated the measurement of stress biomarkers in feathers [35,40]. Bortolotti et al. [86,97] tracked long-term HPA axis activity in birds by measuring the corticosterone concentration in feathers. This method has the advantage of being able to determine current or past stress conditions, even in a dead bird [40].
In the majority of developed countries, human diets are undergoing a gradual shift from being primarily plant-based, with staples such as rice and other grains, to including significant amounts of animal products (e.g., meat, milk, eggs) [98]. Therefore, the demand for broiler chickens is expected to increase, and poultry farmers must prepare for possible challenges. For instance, feather cover plays a critical role in chicken health and welfare, particularly with regard to managing body temperature and protecting the body. Poor feather cover may lead to reduced mating drive and fertility, loss of body weight, and a poorer feed conversion ratio due to inability to regulate body temperature [99]. Feather loss and bald spots in chickens may be caused by environmental stressors (excessive heat or cold, ventilation problems) or imbalances in nutrition. In such cases, where stress threatens bird health, a method is needed to monitor stress-induced body changes in the birds. Measurement of corticosterone in feathers is a suitable alternative to commonly used techniques and can be used to evaluate stress over several weeks or months [86].
Taking feather samples from birds is quick and simple in comparison to blood sampling [40], with the advantages of not requiring expert training, reducing average handling time, and reducing stress on the birds during sampling. Furthermore, blood corticosterone can change quickly in response to environmental stressors or handling, whereas such variation does not occur as quickly in feathers. Another advantage is that feathers can be sampled from dead birds as well as live ones. Feathers can be stored at room temperature, and biomarker measurements taken from feathers are more accurate because of the reduced effect of conditions at the time of sampling [40,100]. A study on pigeons (Columba livia domestica) by Jenni‐Eiermann et al. [101] reported that the amount of corticosterone per millimeter length of feather varied between feather generations in interaction with segment, also finding that levels differed between washed and unwashed feather parts. In a study of broiler chickens, Carbajal et al. [102] found no significant relationship between feather corticosterone levels and physiological variables such as sex, weight, and the presence of fault bars. Lattin et al. [103] stated that the mechanism by which corticosterone is incorporated into feathers is not clear, suggesting that it may be deposited from blood during feather growth and keratinization. Another possibility is that it may be deposited via preen oil or diffusion from the skin surrounding the feather. Steroid hormones are lipophilic and low molecular weight substances that are synthesized by endocrine glands, and are then released into blood circulation. They act both on peripheral target matrices and the CNS. Because of lipophilic properties of steroids, up to approximately 90% of circulating cortisol or corticosterone is bound to carrier proteins such as albumin and corticosteroid binding globulin that can freely diffuse across the cellular membranes [34]. Thus, in birds, circulating corticosterone is incorporated into developing feathers via diffusion from the blood during cell differentiation.
Nails, claws, and hooves
Nails, claws, and hooves are keratinized tissues like hair, and they can similarly be used to assess the level of biological compounds such as cortisol [104]. Biological molecules present in blood can be deposited in these tissues, and their slow growth makes them useful for tracking changes in biomarker levels over month-long periods. Nails grow 3 mm per month in humans [104], whereas Contreras et al. [84] reported that over a three-week period, the front claws of cats grow by 2.4 mm and the hind claws by 1.7 mm. Binz et al. [105] reported different levels of cortisol in the nails of the left and right hands in humans; however, Higashi et al. [106] reported no significant differences between the left and right hands. The advantage of measuring cortisol in fingernails or toenails is that samples can be collected easily and with minimal stress using nail clippers and hoof cutters for humans, chickens, cats, dogs, cattle, sheep, goats, and pigs, then stored for a long time at room temperature.
Scales
Most fish species have scales distributed over their skin. Analysis of biomarkers in scales has been used to evaluate the activity of the hypothalamic–pituitary–interrenal axis in fish [85,107]. Scales can be easily and quickly sampled with negligible injury and stress to the fish, minimizing changes in cortisol concentration due to sampling stress [85,108]. Measuring cortisol in scales is a suitable method for evaluating chronic stress in fish, preferable to measuring it in other matrices such as feces, mucus, gut contents, and fins.
Laboratory techniques
Sample collection
In animal research, samples should be collected and prepared in a manner that ensures data reliability. Before the experiment, animal names, identification numbers, the sampling location, the sampling facility, and the date should be properly recorded. When collecting multiple samples of fluid biomatrices per day, it is important to record the time of sampling due to daily variations in the concentrations of hormones (e.g., cortisol). To minimize risk to researchers, proper health and safety guidelines should be followed, and blood and other biomatrices should be handled properly during collection and storage.
Extraction and detection of stress biomarkers
In order to accurately determine levels of stress biomarkers in biomatrices, extraction procedures should be performed prior to detection by enzyme immunoassay (EIA). Extraction is a way to remove or concentrate the components of interest (e.g., cortisol and corticosterone) from the other components of the biomatrix (e.g., blood, urine, milk, hair, feathers) by applying appropriate solvents. For extracting stress biomarkers from fluid and solid biomatrices, the most widely used techniques are liquid–liquid and solid–liquid extraction with typical solvents such as diethyl ether and methanol. In the standard protocol for biomarker extraction from fluid biomatrices, the sample is diluted with ether (1:10 v/v) and vortexed for 1 min, followed by freezing. The ether supernatant (top layer) is then separated into a new container and saved. This procedure may need to be repeated multiple times for full extraction; each time, the top layers should be combined, and then the collected supernatant should be evaporated to dryness. To ensure reconstitution, the dissolved dried extract should be vortexed well together with an appropriate amount of the assay buffer provided in the EIA kit. Next, the EIA procedure may be continued according to protocol, or the samples may be stored at −20°C until analysis. Finally, the samples should be transferred to a 96-well microtiter plate, and the optical density at 450 nm can be determined using a standard plate reader (the wavelength may differ depending on the EIA kit). Curves for optical standards and experimental samples should be calculated with the analysis software included with the plate reader; alternatively, they can be calculated directly from raw plate data using free data analysis software that can be found online at www.MyAssay.com or other immunoassay analysis websites.
Immunoassay techniques
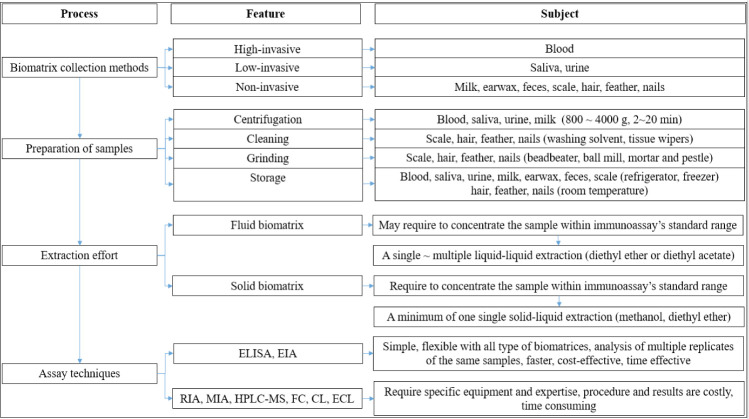
Commonly, the choice of techniques for screening stress biomarkers are not specific matter due to all well-known assays can apply to determine analyte purpose. Accordingly, Meyer et al. [55] mentioned a similar conclusion that the choice of assay is not particularly important as long as it has the requisite specificity for cortisol and a degree of sensitivity appropriate for the size of the samples being analyzed. Various assays can be used for evaluating cortisol and corticosterone concentrations in all types of biomatrices (e.g., high-performance liquid chromatography with mass spectrometry, fluorescence detection, immunoassays); however, immunoassays are the most commonly used method, based on binding between an analyte and an antibody. There are a variety of immunoassay methods, such as enzyme-linked immunosorbent assay (ELISA), radioimmunoassay (RIA), EIA, and the multiplex immunoassay. Each of these has limitations. For instance, RIA is an old assay now rarely used due to the risk posed by radioactive substances, the high level of skill and knowledge required compared with ELISA, and government restrictions due to environmental and safety concerns [33,109]. ELISA is designed to detect a single analyte (e.g., cortisol) in a single biomatrix; thus, for parallel analysis, several experiments are required, which is time- and cost-inefficient and can magnify errors in the results. The multiplex immunoassay can measure multiple biomarkers simultaneously in a single experiment [110] and may utilize flow cytometry, chemiluminescence, or electrochemiluminiscence [111]. The traditional EIA, involving an enzyme attached to an antibody, is capable of measuring one analyte (e.g., cortisol) in one biomatrix (e.g., hair) at a time; however, the multiplex method permits measurement of multiple analytes (e.g., cortisol, estrogen, oxytocin) in the same biomatrix (e.g., hair) at the same time. With traditional singleplex ELISA, it can be complicated to determine how different biomarkers, such as cortisol, cortisone, and estrogen, interact with each other; in comparison, the multiplex immunoassay saves time and expense and produces more biologically informative results. The current review is summarized with regard to measurement of stress hormones in several types of biomatrices (Fig. 1).
Fig. 1. Flow chart showing outline of current review with regard to measurement of cortisol or corticosterone in several types of biomatrices.
ELISA, enzyme-linked immunosorbent assay; EIA, enzyme-immunoassay; RIA, radio-immunoassay; MIA, multiplex immunoassay; HPLC-MS, high-performance liquid chromatography with mass spectrometry; FC, flow cytometry; CL, chemiluminescence; ECL, electrochemiluminescence.
CONCLUSION
Measurement of stress hormones is an efficient method for evaluating stress, as it enables accurate information about physiological conditions to be obtained for living and even dead animals. In studies of chronic stress, the proper selection of biomatrices and biomarkers depends on the type of information required. Solid biomatrices can provide information about how concentrations of major biomarkers of stress (cortisol or corticosterone) have changed over time, making them more suitable than fluid biomatrices for this purpose. Commonly, cortisol or corticosterone extraction from solid biomatrices such as hair and feather, using methanol or diethyl ether that usually used for fluid biomatrices such as plasma and milk. Although, saliva, urine can be assayed after diluting them with sample preparation buffer, the extraction should be considered for accurate screening of cortisol or corticosterone via immunoassay methods. A single solid-liquid extraction procedure by methanol is sufficient to extract cortisol or corticosterone from solid biomatrices. Ideally, multiple (at least 3 times) extractions with diethyl ether are required to maximize the extraction of cortisol or corticosterone from biomatrices. Furthermore, solid biomatrices require less frequent sampling than fluid and semi-solid biomatrices, which saves time and expense and reduces error. At the analysis stage, among a variety of analytical methods used to detect and quantify cortisol and corticosterone in biomatrices, ELISA method is more applicable, highly sensitive, and reliable for screening those analytes than other analytical methods due to the simple procedure (requires no special expertise), analysis of multiple replicates of the same samples on one microtiter plate and faster with results in up to 3 hours, cost-effective, and flexible with biomatrix types. However, the use of a multiplex immunoassay can provide comprehensive details regarding interactions and correlations between various stress biomarkers in a single biomatrix.
Acknowledgements
This paper was supported by the KU Research Professor Program of Konkuk University.
Competing interests
No potential conflict of interest relevant to this article was reported.
Funding sources
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (IPET) and Korea Smart Farm R&D Foundation (KosFarm) through Smart Farm Innovation Technology Development Program, funded by Ministry of Agriculture, Food, and Rural Affairs (MAFRA) and Ministry of Science and ICT (MSIT), Rural Development Administration (RDA) (Grant number: 421013-03).
Availability of data and material
Not applicable.
Authors’ contributions
Conceptualization: Park KH.
Writing-original draft: Ataallahi M, Ghassemi Nejad J.
Writing-reviewing & editing: Ataallahi M, Ghassemi Nejad J, Park KH.
Ethics approval and consent to participate
This article does not require IRB/IACUC approval because there are no human and animal participants.
REFERENCES
- 1.Hirsch MS, Watkins J. A comprehensive review of biomarker use in the gynecologic tract including differential diagnoses and diagnostic pitfalls. Adv Anat Pathol. 2020;27:164–92. doi: 10.1097/PAP.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 2.Dhama K, Latheef SK, Dadar M, Samad HA, Munjal A, Khandia R, et al. Biomarkers in stress related diseases/disorders: diagnostic, prognostic, and therapeutic values. Front Mol Biosci. 2019;6:91. doi: 10.3389/fmolb.2019.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moberg GP. In: The biology of animal stress: basic principles and implications for animal welfare. Moberg GP, Mench JA, editors. Wallingford, Oxon: CABI; 2000. Biological response to stress: implications for animal welfare; pp. 1–21. p. [DOI] [Google Scholar]
- 4.Ewert A, Chang Y. Levels of nature and stress response. Behav Sci. 2018;8:49. doi: 10.3390/bs8050049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi A, Flanigan ME, McEwen BS, Russo SJ. Aggression, social stress, and the immune system in humans and animal models. Front Behav Neurosci. 2018;12:56. doi: 10.3389/fnbeh.2018.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdett S. Exploring the relationships between behavioural responses to stress and performance, carcass composition, energy expenditure and macronutrient oxidation in restrict fed female Yorkshire pigs. Guelph, ON: The University of Guelph; 2019. Ph.D. dissertation. [Google Scholar]
- 7.Ghassemi Nejad J, Sung KI. Behavioral and physiological changes during heat stress in Corriedale ewes exposed to water deprivation. J Anim Sci Technol. 2017;59:1–6. doi: 10.1186/s40781-017-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moberg GP, Mench JA. The biology of animal stress: basic principles and implications for animal welfare. Wallingford, Oxon: CABI; 2000. [DOI] [Google Scholar]
- 9.Victoria Sanz Fernandez M, Johnson JS, Abuajamieh M, Stoakes SK, Seibert JT, Cox L, et al. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol Rep. 2015;3:e12315. doi: 10.14814/phy2.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer JS, Novak MA. In: The international encyclopedia of primatology. Fuentes A, editor. Chichester, UK: Wiley Blackwell; 2017. HPA axis; pp. 597–8. editor. p. [DOI] [Google Scholar]
- 11.Choe SJ, Kim D, Kim EJ, Ahn JS, Choi EJ, Son ED, et al. Psychological stress deteriorates skin barrier function by activating 11β-hydroxysteroid dehydrogenase 1 and the HPA axis. Sci Rep. 2018;8:6334. doi: 10.1038/s41598-018-24653-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin TK, Zhong L, Santiago JL. Association between stress and the HPA axis in the atopic dermatitis. Int J Mol Sci. 2017;18:2131. doi: 10.3390/ijms18102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr Rev. 2017;38:3–45. doi: 10.1210/er.2015-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi H, Arai C, Ikeuchi Y, Yamanaka M, Hirayama T. Effect of growth and parturition on hair cortisol in Holstein cattle. Anim Sci J. 2021;92:e13518. doi: 10.1111/asj.13518. [DOI] [PubMed] [Google Scholar]
- 15.Kovács L, Kézér FL, Bodó S, Ruff F, Palme R, Szenci O. Salivary cortisol as a non-invasive approach to assess stress in dystocic dairy calves. Sci Rep. 2021;11:6200. doi: 10.1038/s41598-021-85666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pompa G, Arioli F, Casati A, Fidani M, Bertocchi L, Dusi G. Investigation of the origin of prednisolone in cow urine. Steroids. 2011;76:104–10. doi: 10.1016/j.steroids.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Gellrich K, Sigl T, Meyer HHD, Wiedemann S. Cortisol levels in skimmed milk during the first 22 weeks of lactation and response to short-term metabolic stress and lameness in dairy cows. J Anim Sci Biotechnol. 2015;6:31. doi: 10.1186/s40104-015-0035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebinghaus A, Knierim U, Simantke C, Palme R, Ivemeyer S. Fecal cortisol metabolites in dairy cows: a cross-sectional exploration of associations with animal, stockperson, and farm characteristics. Animals. 2020;10:1787. doi: 10.3390/ani10101787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weaver SJ, Hynd PI, Ralph CR, Hocking Edwards JE, Burnard CL, Narayan E, et al. Chronic elevation of plasma cortisol causes differential expression of predominating glucocorticoid in plasma, saliva, fecal, and wool matrices in sheep. Domest Anim Endocrinol. 2021;74:106503. doi: 10.1016/j.domaniend.2020.106503. [DOI] [PubMed] [Google Scholar]
- 20.Asano K, Takamatsu E, Numata H, Nitta M, Ishida M. Effect of climatic factors on urinary cortisol and peripheral blood leukocytes in lambs grazing on a semi‐natural grassland in the Hokuriku district of Japan. Anim Sci J. 2021;92:e13536. doi: 10.1111/asj.13536. [DOI] [PubMed] [Google Scholar]
- 21.Choi IH, Park C, Kwak SK, Chung TH. Analysis of plasma cortisol from nursery pigs in outdoor efficacy test for digital content - based approach in animal welfare convergence types. J Environ Sci Int. 2020;29:575–8. doi: 10.5322/JESI.2020.29.5.575. [DOI] [Google Scholar]
- 22.Bahnsen I, Riddersholm KV, de Knegt LV, Bruun TS, Amdi C. The effect of different feeding systems on salivary cortisol levels during gestation in sows on herd level. Animals. 2021;11:1074. doi: 10.3390/ani11041074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim B, Kim HR, Kim KH, Ji SY, Kim M, Lee Y, et al. Effects of acute heat stress on salivary metabolites in growing pigs: an analysis using nuclear magnetic resonance-based metabolomics profiling. J Anim Sci Technol. 2021;63:319. doi: 10.5187/jast.2021.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohan NH, Nath A, Thomas R, Kumar S, Banik S, Das AK, et al. Relationship between plasma, saliva, urinary and faecal cortisol levels in pigs. Indian J Anim Sci. 2020;90:768–72. [Google Scholar]
- 25.Allwin B, Jayathangaraj MG, Palanivelrajan M, Raman M. Evaluation of endogenous faecal cortisol as a non invasive assessment of stress in free ranging wild pigs (Sus scrofa) Indian J Vet Anim Sci Res. 2015;44:89–92. [Google Scholar]
- 26.Mohamed NA, Saad MF, Shukry M, El-Keredy AMS, Nasif O, Van Doan H, et al. Physiological and ion changes of Nile tilapia (Oreochromis niloticus) under the effect of salinity stress. Aquac Rep. 2021;19:100567. doi: 10.1016/j.aqrep.2020.100567. [DOI] [Google Scholar]
- 27.Hudson JM, Anderson WG, Marcoux M. Measurement of cortisol in blow samples collected from free‐swimming beluga whales (Delphinapterus leucas) Mar Mamm Sci. 2021;37:888–900. doi: 10.1111/mms.12779. [DOI] [Google Scholar]
- 28.Ataallahi M, Nejad JG, Takahashi J, Song YH, Sung K, Yun J, et al. Effects of environmental changes during different seasons on hair cortisol concentration as a biomarker of chronic stress in Korean native cattle. Int J Agric Biol. 2019;21:1166–72. [Google Scholar]
- 29.Comin A, Veronesi MC, Montillo M, Faustini M, Valentini S, Cairoli F, et al. Hair cortisol level as a retrospective marker of hypothalamic-pituitary-adrenal axis activity in horse foals. Vet J. 2012;194:131–2. doi: 10.1016/j.tvjl.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Ghassemi Nejad J, Lohakare JD, Son JK, Kwon EG, West JW, Sung KI. Wool cortisol is a better indicator of stress than blood cortisol in ewes exposed to heat stress and water restriction. Animal. 2014;8:128–32. doi: 10.1017/S1751731113001870. [DOI] [PubMed] [Google Scholar]
- 31.Oh S, Hosseindoust A, Ha S, Moturi J, Mun J, Tajudeen H, et al. Dietary fiber for gestating sows during heat stress: effects on reproductive performance and stress level. 2021 doi: 10.21203/rs.3.rs-952458/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weirup L, Schulz C, Seibel H, Aerts J. Scale cortisol is positively correlated to fin injuries in rainbow trout (Oncorhynchus mykiss) reared in commercial flow through systems. Aquaculture. 2021;543:736924. doi: 10.1016/j.aquaculture.2021.736924. [DOI] [Google Scholar]
- 33.Ghassemi Nejad J, Park KH, Forghani F, Lee HG, Lee JS, Sung KI. Measuring hair and blood cortisol in sheep and dairy cattle using RIA and ELISA assay: a comparison. Biol Rhythm Res. 2020;51:887–97. doi: 10.1080/09291016.2019.1611335. [DOI] [Google Scholar]
- 34.Mormède P, Andanson S, Aupérin B, Beerda B, Guémené D, Malmkvist J, et al. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol Behav. 2007;92:317–39. doi: 10.1016/j.physbeh.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Yu DG, Namgung N, Kim JH, Won SY, Choi WJ, Kil DY. Effects of stocking density and dietary vitamin C on performance, meat quality, intestinal permeability, and stress indicators in broiler chickens. J Anim Sci Technol. 202;63:815. doi: 10.5187/jast.2021.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thaxton JP, Stayer P, Ewing M, Rice J. Corticosterone in commercial broilers. J Appl Poult Res. 2005;14:745–9. doi: 10.1093/japr/14.4.745. [DOI] [Google Scholar]
- 37.Nohara M, Tohei A, Sato T, Amao H. Evaluation of response to restraint stress by salivary corticosterone levels in adult male mice. J Vet Med Sci. 2016;78:775–80. doi: 10.1292/jvms.15-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weimer SL, Wideman RF, Scanes CG, Mauromoustakos A, Christensen KD, Vizzier-Thaxton Y. An evaluation of methods for measuring stress in broiler chickens. Poult Sci. 2018;97:3381–9. doi: 10.3382/ps/pey204. [DOI] [PubMed] [Google Scholar]
- 39.Erickson RL, Browne CA, Lucki I. Hair corticosterone measurement in mouse models of type 1 and type 2 diabetes mellitus. Physiol Behav. 2017;178:166–71. doi: 10.1016/j.physbeh.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ataallahi M, Ghassemi Nejad J, Song JI, Kim JS, Park KH. Effects of feather processing methods on quantity of extracted corticosterone in broiler chickens. J Anim Sci Technol. 2020;62:884–92. doi: 10.5187/jast.2020.62.6.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosseindoust A, Oh SM, Ko HS, Jeon SM, Ha SH, Jang A, et al. Muscle antioxidant activity and meat quality are altered by supplementation of astaxanthin in broilers exposed to high temperature. Antioxidants. 2020;9:1032. doi: 10.3390/antiox9111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lennartsson AK, Jonsdottir IH. Prolactin in response to acute psychosocial stress in healthy men and women. Psychoneuroendocrinology. 2011;36:1530–9. doi: 10.1016/j.psyneuen.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Gutiérrez J, Gazzano A, Pirrone F, Sighieri C, Mariti C. Investigating the role of prolactin as a potential biomarker of stress in castrated male domestic dogs. Animals. 2019;9:676. doi: 10.3390/ani9090676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tothova C, Nagy O, Kovac G. Acute phase proteins and their use in the diagnosis of diseases in ruminants: a review. Vet Med. 2014;59:163–80. doi: 10.17221/7478-VETMED. [DOI] [Google Scholar]
- 45.García-Torres S, Cabeza de Vaca M, Tejerina D, Romero-Fernández MP, Ortiz A, Franco D, et al. Assessment of stress by serum biomarkers in calves and their relationship to ultimate pH as an indicator of meat quality. Animals. 2021;11:2291. doi: 10.3390/ani11082291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Y, Arsenault R, Napper S, Griebel P. Models and methods to investigate acute stress responses in cattle. Animals. 2015;5:1268–95. doi: 10.3390/ani5040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandez-Novo A, Pérez-Garnelo SS, Villagrá A, Pérez-Villalobos N, Astiz S. The effect of stress on reproduction and reproductive technologies in beef cattle—a review. Animals. 2020;10:2096. doi: 10.3390/ani10112096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trevisi E, Bertoni G. Some physiological and biochemical methods for acute and chronic stress evaluationin dairy cows. Ital J Anim Sci. 2009;8:265–86. doi: 10.4081/ijas.2009.s1.265. [DOI] [Google Scholar]
- 49.Hughes HD, Carroll JA, Sanchez NC, Richeson JT. Natural variations in the stress and acute phase responses of cattle. Innate Immun. 2014;20:888–96. doi: 10.1177/1753425913508993. [DOI] [PubMed] [Google Scholar]
- 50.Phillips R, Kraeuter AK, McDermott B, Lupien S, Sarnyai Z. Human nail cortisol as a retrospective biomarker of chronic stress: a systematic review. Psychoneuroendocrinology. 2021;123:104903. doi: 10.1016/j.psyneuen.2020.104903. [DOI] [PubMed] [Google Scholar]
- 51.Mendoza SP, Capitanio JP, Mason WA. In: The biology of animal stress: basic principles and implications for animal welfare. Moberg GP, Mench JA, editors. Wallingford: CABI; 2000. Chronic social stress: studies in non-human primates; pp. 227–47. p. [DOI] [Google Scholar]
- 52.Burnard C, Ralph C, Hynd P, Hocking Edwards J, Tilbrook A. Hair cortisol and its potential value as a physiological measure of stress response in human and non-human animals. Anim Prod Sci. 2016;57:401–14. doi: 10.1071/AN15622. [DOI] [Google Scholar]
- 53.Romero LM. Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol. 2004;19:249–55. doi: 10.1016/j.tree.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–17. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyer JS, Novak MA. Minireview: Hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology. 2012;153:4120–7. doi: 10.1210/en.2012-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nedić S, Pantelić M, Vranješ-Đurić S, Nedić D, Jovanović L, Cebulj-Kadunc N, et al. Cortisol concentrations in hair, blood and milk of Holstein and Busha cattle. Slov Vet Res. 2017;54:163–72. doi: 10.26873/SVR-398-2017. [DOI] [Google Scholar]
- 57.Heimbürge S, Kanitz E, Otten W. The use of hair cortisol for the assessment of stress in animals. Gen Comp Endocrinol. 2019;270:10–7. doi: 10.1016/j.ygcen.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 58.Russell E, Koren G, Rieder M, Van Uum S. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 2012;37:589–601. doi: 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Dubey DK, Gnanasekar R. Heat stress in dairy animals: causes, consequences and possible solutions [Internet] engormix. 2008. https://en.engormix.com/dairy-cattle/articles/heat-stress-in-dairy-animals-t34200.htm [cited 2022 Mar 7]
- 60.Trajchev M, Nakov D. Efficiency of installed cooling systems in dairy barns during hot season. J Agric Food Environ Sci. 2017;71:38–45. [Google Scholar]
- 61.Herbut P, Angrecka S, Walczak J. Environmental parameters to assessing of heat stress in dairy cattle—a review. Int J Biometeorol. 2018;62:2089–97. doi: 10.1007/s00484-018-1629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ataallahi M, Park GW, Kim JC, Park KH. Evaluation of substitution of meteorological data from the Korea meteorological administration for data from a cattle farm in calculation of temperature-humidity index. J Clim Change Res. 2020;11:669–78. doi: 10.15531/KSCCR.2020.11.6.669. [DOI] [Google Scholar]
- 63.Park GW, Ataallahi M, Ham SY, Oh SJ, Kim KY, Park KH. Estimating losses in milk production by heat stress and environmental impacts of greenhouse gas emissions in Korean dairy farms. J Anim Sci Technol. 2021 doi: 10.5187/jast.2021.e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.West JW. Effects of heat-stress on production in dairy cattle. J Dairy Sci. 2003;86:2131–44. doi: 10.3168/jds.S0022-0302(03)73803-X. [DOI] [PubMed] [Google Scholar]
- 65.Smyth N. Cortisol secretion in saliva and hair: methodological considerations and relationships with state and trait well-being. London, UK: University of Westminster; 2013. Ph.D. dissertation. [Google Scholar]
- 66.French D. Advances in bioanalytical techniques to measure steroid hormones in serum. Bioanalysis. 2016;8:1203–19. doi: 10.4155/bio-2015-0025. [DOI] [PubMed] [Google Scholar]
- 67.Palme R. Monitoring stress hormone metabolites as a useful, non-invasive tool for welfare assessment in farm animals. Anim Welf. 2012;21:331–7. doi: 10.7120/09627286.21.3.331. [DOI] [Google Scholar]
- 68.Inder WJ, Dimeski G, Russell A. Measurement of salivary cortisol in 2012 – laboratory techniques and clinical indications. Clin Endocrinol. 2012;77:645–51. doi: 10.1111/j.1365-2265.2012.04508.x. [DOI] [PubMed] [Google Scholar]
- 69.Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R. Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia. 2011;166:869–87. doi: 10.1007/s00442-011-1943-y. [DOI] [PubMed] [Google Scholar]
- 70.Fukasawa M, Tsukada H, Kosako T, Yamada A. Effect of lactation stage, season and parity on milk cortisol concentration in Holstein cows. Livest Sci. 2008;113:280–4. doi: 10.1016/j.livsci.2007.05.020. [DOI] [Google Scholar]
- 71.Gormally BMG, Romero LM. What are you actually measuring? A review of techniques that integrate the stress response on distinct time‐scales. Funct Ecol. 2020;34:2030–44. doi: 10.1111/1365-2435.13648. [DOI] [Google Scholar]
- 72.Genther DJ, Laudenslager ML, Sung Y, Blake CR, Chen DS, Lin FR. Assessing systemic stress in otolaryngology: methodology and feasibility of hair and salivary cortisol testing. J Nat Sci. 2015;1:e152. [PMC free article] [PubMed] [Google Scholar]
- 73.Thau L, Gandhi J, Sharma S. Physiology, cortisol [Internet] StatPearls. 2021. https://www.ncbi.nlm.nih.gov/books/NBK538239/ [cited 2022 Mar 7] [PubMed]
- 74.Hansen SH. In: Bioanalysis of pharmaceuticals: sample preparation, separation techniques, and mass spectrometry. Honoré Hansen S, Pedersen-Bjergaard S, editors. Hoboken, NJ: John Wiley & Sons; 2015. Biological samples: their composition and properties, and their collection and storage; pp. 23–30. p. [DOI] [Google Scholar]
- 75.Fernández-Peralbo MA, Luque de Castro MD. Preparation of urine samples prior to targeted or untargeted metabolomics mass-spectrometry analysis. TrAC Trends Analyt Chem. 2012;41:75–85. doi: 10.1016/j.trac.2012.08.011. [DOI] [Google Scholar]
- 76.Niu Z, Zhang W, Yu C, Zhang J, Wen Y. Recent advances in biological sample preparation methods coupled with chromatography, spectrometry and electrochemistry analysis techniques. TrAC Trends Analyt Chem. 2018;102:123–46. doi: 10.1016/j.trac.2018.02.005. [DOI] [Google Scholar]
- 77.Herane-Vives A, Ortega L, Sandoval R, Young AH, Cleare A, Espinoza S, et al. Measuring Earwax Cortisol Concentration using a non-stressful sampling method. Heliyon. 2020;6:e05124. doi: 10.1016/j.heliyon.2020.e05124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palme R. Measuring fecal steroids: guidelines for practical application. Ann N Y Acad Sci. 2005;1046:75–80. doi: 10.1196/annals.1343.007. [DOI] [PubMed] [Google Scholar]
- 79.Shokry E, Pereira J, Marques JG, Júnior, da Cunha PHJ, Noronha Filho ADF, da Silva JA, et al. Earwax metabolomics: an innovative pilot metabolic profiling study for assessing metabolic changes in ewes during periparturition period. PLOS ONE. 2017;12:e0183538. doi: 10.1371/journal.pone.0183538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trumble SJ, Robinson EM, Berman-Kowalewski M, Potter CW, Usenko S. Blue whale earplug reveals lifetime contaminant exposure and hormone profiles. Proc Natl Acad Sci USA. 2013;110:16922–6. doi: 10.1073/pnas.1311418110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zannoni A, Pietra M, Gaspardo A, Accorsi PA, Barone M, Turroni S, et al. Non-invasive assessment of fecal stress biomarkers in hunting dogs during exercise and at rest. Front Vet Sci. 2020;7:126. doi: 10.3389/fvets.2020.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol. 2006;147:255–61. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 83.Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SHM. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 2007;30:E183–91. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- 84.Contreras ET, Vanderstichel R, Hovenga C, Lappin MR. Evaluation of hair and nail cortisol concentrations and associations with behavioral, physical, and environmental indicators of chronic stress in cats. J Vet Intern Med. 2021;35:2662–72. doi: 10.1111/jvim.16283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aerts J, Metz JR, Ampe B, Decostere A, Flik G, De Saeger S. Scales tell a story on the stress history of fish. PLOS ONE. 2015;10:e0123411. doi: 10.1371/journal.pone.0123411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bortolotti GR, Marchant TA, Blas J, German T. Corticosterone in feathers is a long‐term, integrated measure of avian stress physiology. Funct Ecol. 2008;22:494–500. doi: 10.1111/j.1365-2435.2008.01387.x. [DOI] [Google Scholar]
- 87.Meyer J, Novak M, Hamel A, Rosenberg K. Extraction and analysis of cortisol from human and monkey hair. J Vis Exp. 2014;83:e50882. doi: 10.3791/50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meyer JS, Novak MA. Assessment of prenatal stress‐related cortisol exposure: focus on cortisol accumulation in hair and nails. Dev Psychobiol. 2021;63:409–36. doi: 10.1002/dev.22021. [DOI] [PubMed] [Google Scholar]
- 89.Cirimele V, Kintz P, Dumestre V, Goullé JP, Ludes B. Identification of ten corticosteroids in human hair by liquid chromatography–ionspray mass spectrometry. Forensic Sci Int. 2000;107:381–8. doi: 10.1016/S0379-0738(99)00180-2. [DOI] [PubMed] [Google Scholar]
- 90.Koren L, Mokady O, Karaskov T, Klein J, Koren G, Geffen E. A novel method using hair for determining hormonal levels in wildlife. Anim Behav. 2002;63:403–6. doi: 10.1006/anbe.2001.1907. [DOI] [Google Scholar]
- 91.Evans MR, Roberts ML, Buchanan KL, Goldsmith AR. Heritability of corticosterone response and changes in life history traits during selection in the zebra finch. J Evol Biol. 2006;19:343–52. doi: 10.1111/j.1420-9101.2005.01034.x. [DOI] [PubMed] [Google Scholar]
- 92.Azevedo A, Bailey L, Bandeira V, Dehnhard M, Fonseca C, de Sousa L, et al. Age, sex and storage time influence hair cortisol levels in a wild mammal population. PLOS ONE. 2019;14:e0221124. doi: 10.1371/journal.pone.0221124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fourie NH, Brown JL, Jolly CJ, Phillips-Conroy JE, Rogers J, Bernstein RM. Sources of variation in hair cortisol in wild and captive non-human primates. Zoology. 2016;119:119–25. doi: 10.1016/j.zool.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 94.Lutz CK, Meyer JS, Novak MA. Hair cortisol in captive corral-housed baboons. Gen Comp Endocrinol. 2021;302:113692. doi: 10.1016/j.ygcen.2020.113692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Braun U, Michel N, Baumgartner MR, Hässig M, Binz TM. Cortisol concentration of regrown hair and hair from a previously unshorn area in dairy cows. Res Vet Sci. 2017;114:412–5. doi: 10.1016/j.rvsc.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 96.Baier F, Grandin T, Engle T, Edwards-Callaway L. Evaluation of hair characteristics and animal age on the impact of hair cortisol concentration in feedlot steers. Front Vet Sci. 2019;6:323. doi: 10.3389/fvets.2019.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bortolotti GR, Marchant T, Blas J, Cabezas S. Tracking stress: localisation, deposition and stability of corticosterone in feathers. J Exp Biol. 2009;212:1477–82. doi: 10.1242/jeb.022152. [DOI] [PubMed] [Google Scholar]
- 98.Won S, Yoon Y, Hamid MMA, Reza A, Shim S, Kim S, et al. Estimation of greenhouse gas emission from Hanwoo (Korean native cattle) manure management systems. Atmosphere. 2020;11:845. doi: 10.3390/atmos11080845. [DOI] [Google Scholar]
- 99.Kretzschmar-McCluskey V, Fisher C, Van Tuijl O. Ross Technical Notes - a practical guide to managing feather cover in broiler breeder females [Internet] Aviagen; 2014. http://en.aviagen.com/assets/Tech_Center/Ross_Tech_Articles/RossTechNoteFeathering2014-EN.pdf [cited 2022 Mar 7] [Google Scholar]
- 100.Freeman NE, Newman AEM. Quantifying corticosterone in feathers: validations for an emerging technique. Conserv Physiol. 2018;6:coy051. doi: 10.1093/conphys/coy051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jenni‐Eiermann S, Helfenstein F, Vallat A, Glauser G, Jenni L. Corticosterone: effects on feather quality and deposition into feathers. Methods Ecol Evol. 2015;6:237–46. doi: 10.1111/2041-210X.12314. [DOI] [Google Scholar]
- 102.Carbajal A, Tallo-Parra O, Sabes-Alsina M, Mular I, Lopez-Bejar M. Feather corticosterone evaluated by ELISA in broilers: a potential tool to evaluate broiler welfare. Poult Sci. 2014;93:2884–6. doi: 10.3382/ps.2014-04092. [DOI] [PubMed] [Google Scholar]
- 103.Lattin CR, Reed JM, DesRochers DW, Romero LM. Elevated corticosterone in feathers correlates with corticosterone‐induced decreased feather quality: a validation study. J Avian Biol. 2011;42:247–52. doi: 10.1111/j.1600-048X.2010.05310.x. [DOI] [Google Scholar]
- 104.Fischer S, Schumacher S, Skoluda N, Strahler J. Fingernail cortisol–state of research and future directions. Front Neuroendocrinol. 2020;58:100855. doi: 10.1016/j.yfrne.2020.100855. [DOI] [PubMed] [Google Scholar]
- 105.Binz TM, Gaehler F, Voegel CD, Hofmann M, Baumgartner MR, Kraemer T. Systematic investigations of endogenous cortisol and cortisone in nails by LC-MS/MS and correlation to hair. Anal Bioanal Chem. 2018;410:4895–903. doi: 10.1007/s00216-018-1131-6. [DOI] [PubMed] [Google Scholar]
- 106.Higashi T, Yamagata K, Kato Y, Ogawa Y, Takano K, Nakaaze Y, et al. Methods for determination of fingernail steroids by LC/MS/MS and differences in their contents between right and left hands. Steroids. 2016;109:60–5. doi: 10.1016/j.steroids.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 107.Carbajal Brossa A. Cortisol in skin mucus and scales as a measure of fish stress and habitat quality [Ph.D. dissertation] Barcelona, Barcelona: Universitat Autònoma de Barcelona; 2018. [Google Scholar]
- 108.Ghassemi Nejad J, Ataallahi M, Park KH. Methodological validation of measuring Hanwoo hair cortisol concentration using bead beater and surgical scissors. J Anim Sci Technol. 2019;61:41–6. doi: 10.5187/jast.2019.61.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mallick S, Kumar BS, Prakash BS, Aggrawal A, Pandita S. Development and validation of a simple, sensitive enzyme immunoassay for quantification of androstenedione in bull plasma. J Anim Sci Technol. 2015;57:1–5. doi: 10.1186/s40781-014-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–23. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci. 2008;63:879–84. doi: 10.1093/gerona/63.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]