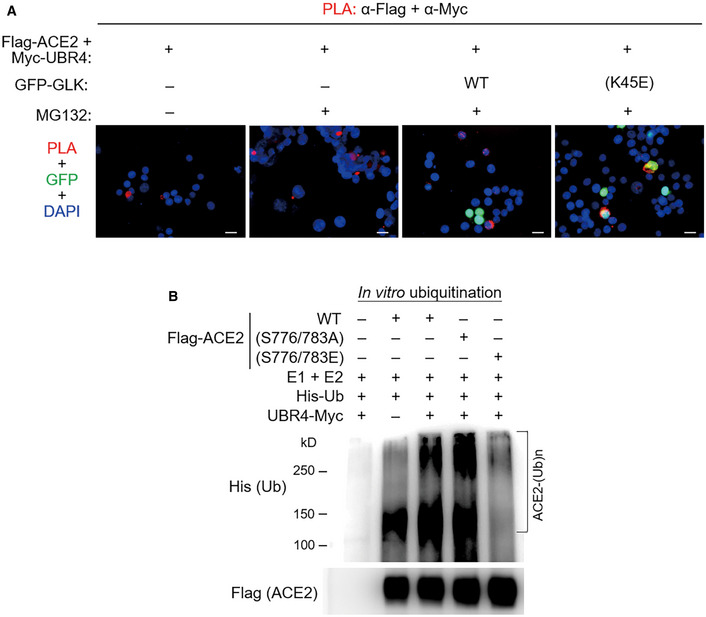
Figure EV3. GLK‐induced ACE2 phosphorylation blocks the ACE2‐UBR4 interaction and subsequent ACE2 ubiquitination.
-
AIn situ PLA assays of the interaction between Flag‐tagged ACE2 and Myc‐tagged UBR4 proteins in HEK293T cells. Cells were co‐transfected with Flag‐ACE2 and Myc‐UBR2 plus either GFP‐GLK (green) or GFP‐GLK (K45E) kinase‐dead mutant (green) plasmids. Nuclei were stained with DAPI. Red dots represent direct interaction signals. Yellow color represents the co‐existence of PLA signal (red) and GLK (K45E) mutant protein (green). Original magnification, ×200. Scale bars, 20 μm.
-
BIn vitro ubiquitination assays using Myc‐tagged UBR4 and Flag‐tagged ACE2 proteins with E1 ubiquitin‐activating enzyme, E2 ubiquitin‐conjugating enzyme, and His‐tagged ubiquitin. ACE2 ubiquitination and ACE2 were detected by immunoblotting with anti‐His and anti‐Flag antibodies, respectively. Data shown are representative results of three independent experiments.
