Table 1.
Optimization of Amination Conditionsa
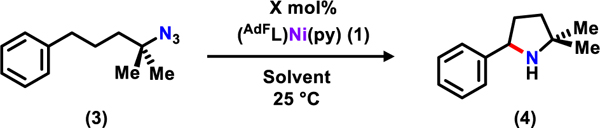
| ||||
|---|---|---|---|---|
| Entry | Catalyst Loading (mol%) | Solvent | Time | Yield (%)d |
| 1 | 10b | C6D6 | 10 min | > 99 |
| 2 | 5b | C6D6 | 20 min | > 99 |
| 3 | 2b | C6D6 | 40 min | 97 |
| 4 | 1b | C6D6 | 90 min | 96 |
| 5 | 0.1 | C6D6 | 48 h | 73 |
| 6 | 1b | Hexanes | 90 min | 95 |
| 7 | 1b | Et2O | 90 min | 96 |
| 8 | 1b | (d8-)Toluenee | 90 min | 95 |
| 9 | 1b | d8-THF | 90 min | 94 |
| 10 | 10 | d6-DMSO | N/Ac | 0 |
| 11 | 10 | d4-Methanol | N/Ac | 0 |
| 12 | 10 | DCM | N/A | 0 |
Reaction were conducted on 0.2 mmol scale, see SI for details of the experiments.
Regeneration of 1 by 19F NMR spectroscopy.
Catalyst decomposition.
Yields were determined by 1H NMR spectroscopy using tetrakis(trimethylsilyl)silane as internal standard.
Both proteo- and deutero-toluene were tested as solvent, and no difference was observed.
