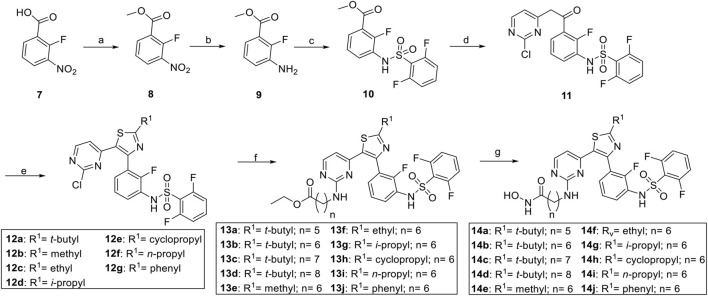
SCHEME 1.
Synthesis of compounds 14a–14j. a Reagents and conditions: (A) SOCl2, MeOH, reflux, 1 h, 87%; (B) 10% Pd/C, H2, rt, 100%, (C) 2,6-difluorobenzenesulfonyl chloride, pyridine, DCM, rt, overnight, 98%; (D) 2-chloro-4-methylpyrimidine, LiHMDS, 0°C to rt, 1 h, 92%; (E) NBS, 2,2,2-trimethylthioacetamide, DMA, rt to 60°C, 1 h, 30%–44%; (F) amines, CsCO3, NMP, 60°C, 17 h, 42%–60%; (G) NH2OH/KOH in MeOH, 0°C, 0.25 h, 50%–74%.