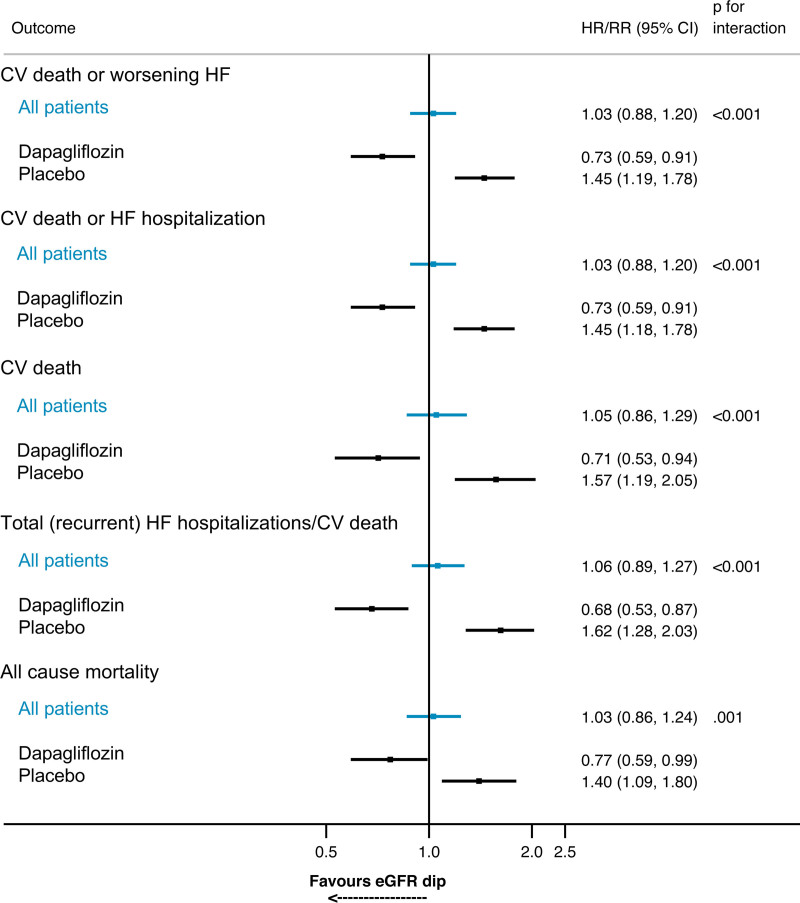
Figure 2.
Risk of prespecified outcomes for patients experiencing a >10% decline in eGFR between baseline and day 14 compared with patients not experiencing a >10% decline in eGFR within each randomized treatment group. Follow-up is for outcomes occurring after 14 days (landmark analysis). Models are stratified by diabetes status and adjusted for baseline estimated glomerular filtration rate (eGFR) and history of heart failure (HF) hospitalization (HF hospitalization for each outcome apart from all-cause mortality). Analysis for all patients includes adjustment for randomized treatment. For example, between day 14 and the end of the study, the risk of the primary outcome (cardiovascular [CV] death or worsening HF) was 45% higher in patients randomized to placebo who experienced a >10% decline in eGFR between baseline and day 14 compared with placebo-treated patients who did not experience this decline. Conversely, the risk of the primary outcome was 27% lower among patients randomized to dapagliflozin who experienced a >10% decline in eGFR compared with dapagliflozin-treated patients who did not experience a >10% decline in eGFR between day 0 and 14. HR indicates hazard ratio; and RR, rate ratio.