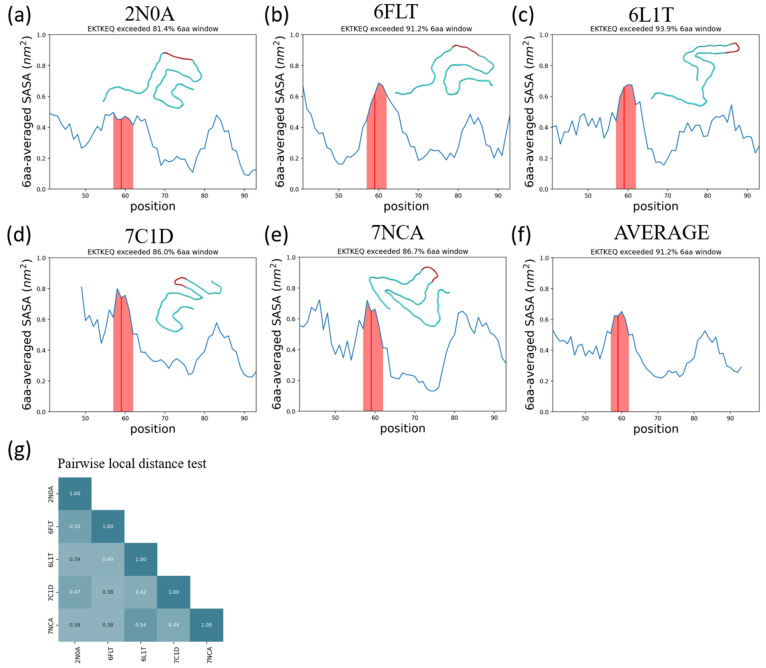
Figure 8.
The α-synuclein epitope, EKTKEQ, is found to be highly exposed in five structurally distinct fibrils. (a–e) Averaged SASA as a function of residue position. A rolling average window of 6 amino acids was applied. The window that contains EKTKEQ (residues 57–62), as indicated by the red line, exceeds more than 80% of the other windows in all fibril structures analyzed. The shaded region contains the rolling average values for residues 57–62. In each panel, a single chain of each fibril structure is aligned and rendered to show their structure heterogeneity. (f) The average SASA across all 5 fibrils. The epitope region has the highest average SASA across the whole structured sequence. (g) The pairwise local distance test (lddt)74 shows that the analyzed fibrils are all mutually dissimilar.