Abstract
Investigation of the nutritional as well as trace elements of a wild leafy vegetable, Acalypha alnifolia, and evaluation of the analgesic, anti-inflammatory, and antipyretic properties of acetone and methanol leaf extracts are the main objectives of the present study. The powdered A. alnifolia leaf sample was subjected to nutritional and mineral analysis. Plant leaves were extracted (using the Soxhlet apparatus) as successive solvent extractions. The extract doses of 200 and 400 mg/kg of acetone and methanol extracts were used for pharmacology study. The analgesic, anti-inflammatory, and antipyretic experiments were carried out by using animal models. The obtained result proves that the plant possesses essential nutritive values and useful biological properties. The higher dose of acetone extract has significant potency when compared with methanol extract at p < 0.005. On the whole, the plant is rich in minerals and has good biological properties; hence, this plant is suggested for cultivation and regular use for nutritional supplement.
Keywords: Acalypha alnifolia, Anti-inflammatory property, Leafy vegetable, Minerals, Nutrition
1. Introduction
Understanding the chemical composition and potential biological properties of plant extracts is essential for their further use in the food industry [1]. The concentration of essential and nonessential heavy metals in medicinal plants beyond permissible limits is a matter of great concern to ensure public safety all over the world [2]. Plants are also known to have high amounts of essential nutrients, vitamins, minerals, fatty acids, and fiber [3]. Minerals are required for normal growth, activities of muscles, skeletal development (such as calcium), cellular activity, oxygen transport (copper and iron), chemical reactions in the body, intestinal absorption (magnesium), fluid balance, and nerve transmission (sodium and potassium), as well as the regulation of the acid–base balance (phosphorus) [4].
Previous epidemiological studies have consistently shown that consumption of fruits and vegetables is associated with reduced risk of chronic diseases, such as cardiovascular diseases and cancers [5], neurodegenerative diseases [6], and inflammation [7]. Studies originally showed that optimal intakes of elements, such as sodium, potassium, magnesium, calcium, manganese, copper, zinc, and iodine, can reduce individual risk factors including those related to cardiovascular diseases among humans and animals [8].
The classical signs of acute inflammation are pain, heat, redness, swelling, and loss of function. Inflammation serves to destroy injurious and reconstitute the damaged tissue. Normally, the infected or damaged tissue initiates the enhanced formation of proinflammatory mediators that increase the synthesis of prostaglandin E2 near peptic hypothalamus areas, thereby triggering the hypothalamus to elevate the body temperature [9]. Hence, pain, inflammation, and temperature increase are regarded as indications of infection or injury.
Analgesic drugs inhibit the mediators and alleviate symptoms so patients could feel better. However, patients cannot continue taking analgesic drugs for long. The major reasons for discontinuation of opioid analgesic treatment are gastrointestinal and central nervous system (CNS) side effects [10]. Indeed, in some patients pain alone may cause nausea and vomiting. Therefore, there is a need to develop a new class of analgesic drugs with improved benefit–risk profiles [11].
The development of drugs for nutritional substitution, especially those with potent analgesic and anti-inflammatory properties with lesser side effects, is necessary in this situation. In recent years, the search for drugs and dietary supplements derived from plants has picked up pace in drug discovery. Therefore, a meticulously designed and dose-based experimental evaluation on this encouraging subject would bring out a new input in the realm of naturally present anti-inflamogens to substitute for existing synthetic agents [12]. The plant Acalypha alnifolia is viewed as a vegetable by the inhabitants of Nilgiri District [13]. The leaf paste of the plant is an ingredient of the traditional formulation used to cure prickles by the Hoorali tribe of Sathyamangalam forest [14].
A. alnifolia has a significant antioxidant property that is coincidentally comparable with the quantity of phenolics; hence, it is expected to be of pharmacological value [15]. In the present study, we evaluated the analgesic, anti-inflammatory, and antipyretic properties as well as the mineral and nutritional properties of this plant.
2. Materials and methods
2.1. Plant collection and extraction of plant material
A. alnifolia was collected from the Bharathiar University campus. The voucher specimen was deposited and then identified and authenticated by the Botanical Survey of India, Southern Circle, Coimbatore, India (authentication number BSI/SRC/5/23/2011-12/Tech.-1137). The leaves were separated from the plant, washed in running tap water, and shade-dried. Then it was powdered and packed in small thimbles separately and extracted successively with different solvents such as petroleum ether, chloroform, acetone, and methanol in the increasing order of polarity using the Soxhlet apparatus. The extract obtained was dried and used for further assessments.
2.2. Nutritional studies
The powdered plant leaf was taken for nutritional studies.
2.2.1. Estimation of carbohydrate and protein
The plant’s carbohydrate content was estimated following the method described by Sadasivam and Manickam [16], using glucose as the standard. The protein content was estimated as described by Lowry et al [17], using bovine serum albumin as the standard.
2.2.2. Estimation of amino acid
The amino acids in the samples were determined following the procedure described by Ishida et al [18]. Here, the gradient system was followed for the effective separation of amino acids. The oven temperature was maintained at 60°C and was programmed for 60 minutes. The amino acid analysis was done with the nonswitching flow method and fluorescence detection after postcolumn derivatization with o-phthaldehyde. The amino acid standard (Sigma Chemical Co., St. Louis, MO, USA) was also run to calculate the concentration of amino acids in the sample. The amount of each amino acid is expressed as g/100 g protein.
2.2.3. Estimation of phosphorous
To determine the phosphorus content, 1 mL of triple acid-digested extract was added to 5 mL ammonium molybdate–sulfuric acid reagent. A blue color was developed by adding six drops of stannous chloride solution. The intensity of the (blue) color was measured at 650 nm in a spectrophotometer. The phosphorus content was determined using the method of Dickman and Bray [19].
where F represents factor (100 = how much ppm).
Estimation of manganese (Mn), iron (Fe), copper (Cu), zinc (Zn), and boron (B) contents using atomic absorption spectroscopy was performed as per the method described by Kalra [20]. For Si and N estimation, the method described by the Association of Analytical Chemists (AOAC 1990) was followed. The wavelength settings for sensitize the elements Cu, Fe, Mg, Zn, Mn, and B are 324.7, 248.3, 285.2, 213, 257.6, and 249.7 nm, respectively.
2.2.4. Calcium and magnesium estimation
Five milliliters of the sample solution was pipetted out into a beaker [21]. Then, 5 mL KCN solution and 10 mL ammonium chloride–ammonium hydroxide buffer were added, followed by two drops Solo chrome black 1 indicator titrated against EDTA. At the end point, the wine-red color of the solution changes to pure blue. Five milliliters of the blank solution was also titrated as described above.
2.2.5. Estimation of potassium and sodium
Double acid treatment was done with nitric acid and perchloric acid (3:1). First, 0.3 g of dry sample was weighed in a conical flask [22]. Then, 5 mL of double acid was added. The mixture was digested, and the residue was washed by water and filtered through an ordinary filter paper, and the filtrate was made to 100 mL. This solution was taken as A. Five milliliters of the above solution was taken and made up to 50 mL using distilled water. This was tagged as solution B and used for potassium (K) estimation.
For sodium estimation, 0.3 g of dry sample was weighed in a conical flask, after which 5 mL of double acid was added. A glass funnel was placed and the mixture was digested until the solution became clear. The residue was washed by water and filtered through an ordinary filter paper, and the filtrate was made to 100 mL. Next, 5 mL of this solution was taken and made up to 50 mL using distilled water.
where F denotes factor (100 = how much ppm).
2.2.6. Estimation of molybdenum (molybdenum dithiol colorimetric method)
The color was measured on a spectrophotometer at 680 nm using a red filter and sample blank as reference. From a prepared calibration curve, the amount of Mo in the digest was determined.
2.2.7. Estimation of crude fiber
In the Weendes method, when the sample is subjected to acid and alkali digestion, we obtain a residue comprising ash (mineral matter) of the feed and the resistant fraction of carbohydrate. When the residue is ignited the organic matter gets oxidized, leaving the inorganic residue or ash. Thus, the difference in weight of the residue before and after ashing gives the weight of the crude fiber.
2.3. In vivo studies
2.3.1. Animals used
Adult Wister albino rats weighing between 150 and 200 g and adult Swiss albino mice weighing between 25 and 30 g (for analgesic experiment only) of either sex were used for the studies. The study was carried out with consent from the Committee for the Purpose of Control, Supervision on Experimental Animals (CPCSEA) (688/02/C/CPCSEA) and the Institutional Animal Ethics Committee (IAEC), Proposal number NCP/IAEC/PG/2010-04.
2.3.2. Acute toxicity
The animals were divided into control and test groups containing six animals each. The test groups got marked doses of acetone and methanol extracts orally up to 2000 mg/kg and observed for mortality until 48 h as per the Organization for Economic Co-operation and Development guidelines. As there was no mortality observed, the doses fixed as 1/10 and 1/5 of the higher dose were selected, and each extract was taken as a 1:1 ratio with 10% carboxymethyl cellulose for the experimental study.
2.3.3. Analgesic activity
2.3.3.1. Tail flick method
Before the study, Swiss albino mice were screened for sensitivity test by placing the tip of the tail on the radiant heat source. Animals that showed a tendency to withdraw its tail in 5 seconds were excluded from the study. The selected animals were divided into six groups of six mice each. Each animal of the groups received one of the following extracts: 200 and 400 mg/kg of acetone and methanol extracts, pentazocine 10 mg/kg (intraperitoneally). Analgesia was assessed with the tail flick apparatus, an analgesiometer [23].
2.3.3.2. Hot-plate reaction time in mice
Mice were screened by placing them on a hot-plate maintained at 55 ± 1°C, and the reaction time (in seconds) for hind paw licking or jumping were recorded [24]. Mice with a reaction time of within 5 seconds were selected. Pentazocine (10 mg/kg, intraperitoneally) was used as the standard. The time for hind paw licking or jumping on the heated plate of analgesiometer was taken as the reaction time.
Analgesic activity was determined by comparing with the control group. For both assessments, the basal reaction time was measured initially, and another set of four measures were taken at 30-, 60-, 90- and 120-minute intervals; the reaction of the animals was regarded as the postdrug reaction time. A cutoff period of 15 seconds was observed to prevent tissue damage on the tail of the animals [25].
2.3.4. Anti-inflammatory experiments
The different groups were treated with acetone and methanol extracts of A. alnifolia (200 and 400 mg/kg, p.o.) indomethacin (10 mg/kg, p.o.). The paw volume of Wister albino rats were measured at 0, 1, 2, 3, and 4 hours after carrageenan and egg albumin injection according to the method described by Winter and Portar [26].
2.3.4.1. Carrageenan-induced paw edema in rats
The animals were pretreated with the extract 1 hour before the administration of carrageenan. Acute inflammation was produced via the subplanter administration of 0.1 mL (1%, w/v) carrageenan in normal saline in the right paw of the rats. Change in paw volume was measured with a Vernier caliper, and the anti-inflammatory activity was calculated [27].
2.3.4.2. Egg albumin-induced paw edema
The phlogistic agent used in this study was fresh undiluted egg albumin. Inflammation of the hind paw was induced by an injection of 0.1 mL fresh undiluted egg albumin into the subplantar surface of the animals’ right hind paw. Paw diameters were measured with the aid of a Vernier caliper before and after the administration of egg albumin for 4 hours at 1-hour intervals [28].
The ratio of the anti-inflammatory effect of each was calculated using the following equation [29]:
where
Ct = paw circumference at time t
C0 = paw circumference before carrageenan/egg albumin injection
Ct–C0 = edema
2.3.5. Induction of fever by yeast-induced pyrexia
Antipyretic activity on albino rats was studied with fever induced by 20% (20 mL/kg) Brewer’s yeast. Albino rats were fed uniformly up to 24 hours, and rectal temperature was measured. After 18 hours of yeast injection, rats that exhibited a rise in temperature of at least 0.7°C were taken for further study [30]. Paracetamol (150 mg/kg) was taken as the standard drug. Temperature was measured at every 1 hour up to 4 hours.
2.4. Statistics
The obtained results were expressed as mean ± standard error and compared with those of the corresponding control group by applying one-way analysis of variance. The significance of each in vivo experiment and comparison with the control group was assessed using Dunnet’s t test.
3. Results
3.1. Nutrition analysis
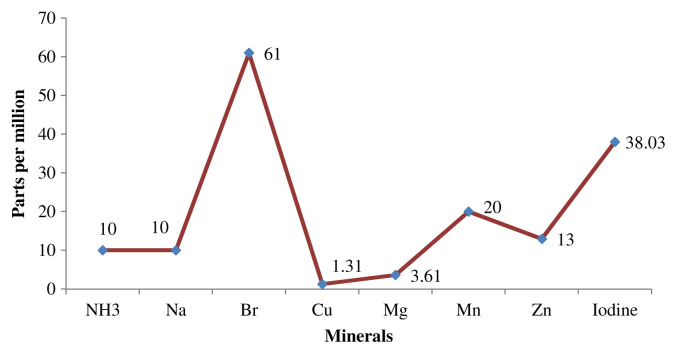
A healthy diet contains carbohydrate, proteins, and minerals, which are mostly obtained from natural foods. In the present study, the wild leafy vegetable contains all the components but its carbohydrate content is much less than that of proteins and minerals, which is sufficient to fulfill the metabolic needs of an average person. The major dietary components are quantified and shown in Table 1 and Fig. 1.
Table 1.
Quantification of nutritional components in Acalypha alnifolia leaf.
| A. alnifolia | Carbohydrate (mg/g) | Protein (mg/kg) | Minerals (ppm) | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Fe | N | K | Ca | P | |||
| Leaf | 0.055 | 2.19 | 1.03 | 18,900 | 8900 | 1800 | 230,000 |
Fig. 1.
The micronutrient mineral content of Acalypha alnifolia leaf.
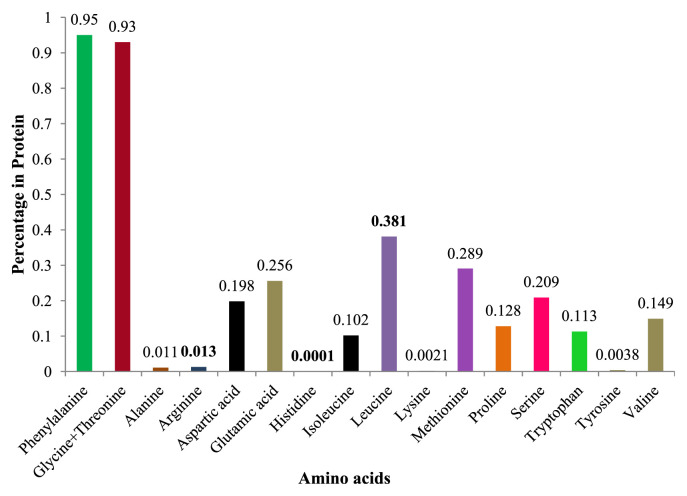
The entire nutritional source could contain a large quantity of amino acids because amino acids are the building blocks of proteins and act as catalyst in many metabolic processes. In this vein, essential amino acids were screened from the result, and it was noted that the quantity of phenylalanine and glycine + threonine (followed by leucine and methionine) was higher than that of others. The reports on screened amino acids are shown in Fig. 2.
Fig. 2.
Amino acid profile of Acalypha alnifolia leaf.
3.2. Analgesic—tail flick experiment
Analgesics act to inhibit pain-transmitting neurons; hence, the rats could tolerate the heat longer compared with the rats in the control group (results are shown in Table 2). In this experiment, tail withdrawal (in seconds) was measured every 30 minutes up to 2 hours. All four doses have significant pain-reducing effects when compared to the control groups at p < 0.05 and p < 0.005 levels, as higher doses of acetone and methanol (400 mg/kg) have good analgesic properties and are almost as good as analogues. The extract potency is good and effective in higher doses.
Table 2.
Tail flick analgesic effect of A. alnifolia leaf extracts in mice.
| Treatment | Response (s)/time (min) | ||||
|---|---|---|---|---|---|
|
| |||||
| 0 | 30 | 60 | 90 | 120 | |
| Control | 5.86 ± 0.33 | 6.76 ± 0.32 | 6.38 ± 0.72 | 7.08 ± 0.55 | 6.8 ± 0.24 |
| Pentazocine (10 mg/kg) | 5.5 ± 0.12 | 5.83 ± 0.34 | 6.18 ± 0.22 | 8.28 ± 0.14 | 10.48 ± 0.56*** |
| AAAE (200 mg/kg) | 6.02 ± 0.78 | 6.12 ± 0.24 | 7.23 ± 0.32 | 7.65 ± 0.53 | 8.39 ± 0.27*** |
| AAAE (400 mg/kg) | 5.88 ± 0.42 | 6.88 ± 0.62 | 7.67 ± 0.77 | 8.14 ± 0.32 | 9.31 ± 0.32*** |
| AAME (200 mg/kg) | 5.69 ± 26 | 5.98 ± 0.13 | 6.49 ± 0.25 | 7.22 ± 0.39 | 7.82 ± 0.43** |
| AAME (400 mg/kg) | 5.44 ± 0.25 | 6.42 ± 0.18 | 7.08 ± 0.28 | 7.63 ± 0.06 | 8.28 ± 0.13*** |
Data are expressed as mean ± standard error of the mean.
p < 0.05,
p < 0.005,
p < 0.001.
AAAE = Acalypha alnifolia acetone extract; AAME = Acalypha alnifolia methanol extract.
3.3. Analgesic—hot-plate method
In this experiment, the result shows that the higher dose of acetone showed good pain-tolerating effects compared with other doses. This is more effective in consequent observation periods. The comparison with the control group at the significance level of p < 0.05 revealed that the methanol extract at 400 mg/kg performed much better. The results, expressed as seconds of heat tolerance of each group (mean), are shown in Table 3.
Table 3.
Hot-plate analgesic effect of Acalypha alnifolia leaf extracts in mice.
| Treatment | Response (s)/time (min) | ||||
|---|---|---|---|---|---|
|
| |||||
| 0 | 30 | 60 | 90 | 120 | |
| Control | 3.34 ± 0.33 | 5 ± 0.57 | 5.67 ± 0.66 | 6.34 ± 0.34 | 5.33 ± 0.88 |
| Pentazocine (10 mg/kg) | 5.67 ± 0.32 | 6 ± 0.57 | 8 ± 0.57 | 11 ± 0.57 | 8.33 ± 0.82 |
| AAAE (200 mg/kg) | 4.33 ± 0.88 | 5.33 ± 0.88 | 7.10 ± 0.57 | 7.33 ± 0.33 | 6.66 ± 0.66 |
| AAAE (400 mg/kg) | 5.33 ± 0.66 | 5.96 ± 0.53 | 7.21 ± 0.57 | 10.33 ± 0.88* | 8.0 ± 0.57 |
| AAME (200 mg/kg) | 4.66 ± 0.33 | 6.33 ± 0.33 | 7.66 ± 0.33 | 7.15 ± 0.57 | 6.66 ± 0.33 |
| AAME (400 mg/kg) | 5.0 ± 0.57 | 6.66 ± 0.66 | 7.66 ± 0.88 | 8.0 ± 0.57* | 6.33 ± 0.33 |
Data are expressed as the mean ± standard error of the mean.
p < 0.05.
AAAE = Acalypha alnifolia acetone extract; AAME = Acalypha alnifolia methanol extract.
3.4. Anti-inflammatory—carrageenan-induced paw edema
For each animal, edema was expressed as % increase in paw volume after carrageenan injection relative to the preinjection value, considered as 100%. The paw volume of each animal was measured before carrageenan injection, which was considered as 0 hour. After the injection, the paw volume was measured every hour up to 4 hours for the calculation. Significance at p < 0.005 was recorded in the higher dose of acetone extract. In this result, the percentage of inhibition is taken for concluding the effective extract and dose (Table 4). The percentage of inhibition in acetone extract (400 mg/kg) is considerably better when compared with the control group in the 4th hour.
Table 4.
Anti-inflammatory effects of Acalypha alnifolia leaf extracts—carrageenan-induced paw edema in rats.
| Treatment | Paw volume (mm)/time in (h) | % inhibition | |||
|---|---|---|---|---|---|
|
| |||||
| 0 h | 1 h | 2 h | 4 h | ||
| Control | 4.58 ± 0.05 | 5.60 ± 0.07 | 5.61 ± 0.06 | 5.47 ± 0.03 | — |
| STD indomethacin | 4.61 ± 0.12 | 5.33 ± 0.06 | 5.04 ± 0.05 | 4.68 ± 0.08 | 92.13 |
| AAAE (200 mg/kg) | 4.57 ± 0.07 | 5.63 ± 0.11 | 5.29 ± 0.07 | 5.06 ± 0.09* | 44.94 |
| AAAE (400 mg/kg) | 4.87 ± 0.05 | 5.74 ± 0.03 | 5.16 ± 0.04 | 5.03 ± 0.03** | 82.02 |
| AAME (200 mg/kg) | 4.87 ± 0.02 | 5.65 ± 0.07 | 5.62 ± 0.20 | 5.56 ± 0.18 | 22.47 |
| AAME (400 mg/kg) | 4.37 ± 0.03 | 5.57 ± 0.07 | 5.28 ± 0.04 | 4.75 ± 0.06 | 57.30 |
Data are expressed as the mean ± standard error of the mean.
p < 0.05,
p < 0.005.
AAAE = Acalypha alnifolia acetone extract; AAME = Acalypha alnifolia methanol extract; STD = standard.
3.5. Anti-inflammatory—egg albumin induced paw edema
Egg albumin, acting as a phlogistic agent, causes inflammation. In this experiment, the methanol extract (400 mg/kg) gives a much better inhibition percentage, followed by acetone extract (400 mg/kg). They are compared with standard indomethacin and control; the results are shown in Table 5. On the 4th hour, the inhibition was better and the percentage inhibition was measured as 74.41% and 72.09%, respectively. The higher doses of each extracts are analogues but with lesser diminution in acetone extract.
Table 5.
Anti-inflammatory effects of Acalypha alnifolia leaf extracts—egg albumin-induced paw edema in rats.
| Treatment | Paw volume (mm)/time (h) | % inhibition | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0 h | 1 h | 2 h | 3 h | 4 h | ||
| Control | 5.43 ± 0.23 | 6.38 ± 0.07 | 5.80 ± 0.07 | 5.58 ± 0.08 | 5.55 ± 0.36 | — |
| STD indomethacin | 3.63 ± 0.26 | 4.65 ± 0.59 | 4.13 ± 0.04 | 3.68 ± 0.80 | 3.68 ± 0.03* | 88.37 |
| AAAE (200 mg/kg) | 4.24 ± 0.39 | 5.64 ± 0.65 | 5.48 ± 0.47 | 5.34 ± 0.41 | 4.67 ± 0.08 | 27.90 |
| AAAE (400 mg/kg) | 4.13 ± 0.15 | 5.18 ± 0.15 | 5.3 ± 0.02 | 5.96 ± 0.21 | 4.44 ± 0.32* | 72.09 |
| AAME (200 mg/kg) | 4.29 ± 0.64 | 4.91 ± 0.68 | 4.93 ± 0.59 | 4.40 ± 0.64 | 4.67 ± 0.59 | 11.62 |
| AAME (400 mg/kg) | 4.31 ± 0.18 | 4.72 ± 0.17 | 5.3 ± 0.18 | 4.93 ± 0.19 | 4.45 ± 0.45* | 67.44 |
Data are expressed as the mean ± standard error of the mean.
p < 0.05.
AAAE = Acalypha alnifolia acetone extract; AAME = Acalypha alnifolia methanol extract; STD = standard.
3.6. Antipyretic—yeast-induced pyrexia experiment
The changes in temperature in each group are shown in Table 6. The plant extracts have a good antipyretic activity in the 4th hour. Higher doses of each extract, in particular, showed a good body heat reducing power.
Table 6.
Antipyretic activity of Acalypha alnifolia leaf extracts in rats.
| Treatment | Rectal temperature (°C) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| After yeast injection | After drug administration | |||||
|
|
|
|||||
| 0 h | 18 h | 1 h | 2 h | 3 h | 4 h | |
| Control | 37.2 ± 0.63 | 38.2 ± 0.32 | 38.8 ± 0.77 | 38.2 ± 0.24 | 38.2 ± 0.15 | 38.1 ± 0.63 |
| Paracetamol (150 mg/kg) | 36.9 ± 0.92 | 37.7 ± 0.76 | 37.4 ± 0.12 | 36.9 ± 0.43 | 37.1 ± 0.62 | 36.9 ± 0.92* |
| AAAE (200 mg/kg) | 37.2 ± 0.71 | 37.9 ± 0.43 | 37.6 ± 0.44 | 37.5 ± 0.28 | 37.4 ± 0.26 | 37.4 ± 0.71* |
| AAAE (400 mg/kg) | 37.1 ± 0.84 | 38.1 ± 0.33 | 37.4 ± 0.16 | 37.9 ± 0.57 | 37.2 ± 0.27 | 37.1 ± 0.84* |
| AAME (200 mg/kg) | 37.3 ± 0.17 | 37.8 ± 0.91 | 37.9 ± 0.87 | 37.3 ± 0.11 | 37.6 ± 0.12 | 37.5 ± 0.17* |
| AAME (400 mg/kg) | 36.8 ± 0.23 | 37.3 ± 0.45 | 36.7 ± 0.12 | 36.8 ± 0.22 | 36.6 ± 0.37 | 36.9 ± 0.23* |
Data are expressed as the mean ± standard error of the mean.
p < 0.05.
AAAE = Acalypha alnifolia acetone extract; AAME = Acalypha alnifolia methanol extract.
4. Discussion
Many natural health experts subscribe to the belief that inflammation is actually at the heart of all diseases, and dietary choices play a big role as well. According to the eminent physician Andrew Weil, “Following an anti-inflammatory diet can help counteract the chronic inflammation that is a root cause of many serious diseases, including those that become more frequent as people age. It is a way of selecting and preparing foods, based on science that can help people achieve and maintain optimum health over their lifetime.” The mineral can have a dramatic impact on inflammation throughout the body. As the trigger to many chronic diseases including cardiovascular disease, inflammation is often only directly dealt with when people begin to feel its effects.
A good knowledge of the chemical composition and potential biological properties of vegetables and fruits is an essential step in for their further use in the food industry. By concentrating on such foods, we can reduce their potential harmful effects on health in the future, and now is the right time to focus on functional foods. Hence, with this new research, learning how specific foods can influence the inflammatory process is the best strategy for containing it, and reducing disease risks and inflammation throughout the body without drugs can be achieved [31].
In view of the importance of these macro- and micro-mineral elements in human nutrition, animals and plants and their metabolic interrelationships that influence other vital factors are needed for the survival of living organisms such as enzymes and antioxidants [32]. Proteins can also be very important in communication in the body, with amino acids being the monomers of each protein. The body requires 20 different amino acids, of which eight are referred to as essential amino acids that cannot be synthesized by the human body. Animals and plants have been manufacturing proteins for which these essential amino acids are acting as monomers.
Isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine are some of the essential amino acids present in the A. alnifolia leaf. Significantly, this plant is rich in threonine and leucine—suggesting that this leafy vegetable can provide what the human body needs. This vegetable also contains a very small amount of histidine, which is an essential amino acid for babies but not for adults. The essential amino acids that our body cannot produce are abundantly present in this leafy vegetable.
4.1. Contribution of minerals in health
Sodium micronutrient is present usually as sodium chloride (NaCl). Sodium, which controls the body’s water balance and plays a role in muscle contraction, is also present in this plant (which could be as low as 10 ppm). Potassium deficiency affects the collecting tubules of the kidney, resulting to the inability to concentrate urine, and also causes alterations of gastric secretions and intestinal motility [33].
A diet high in calcium and magnesium can lead to sodium loss. Deficiency or excess may cause congestive heart failure. Na and K are of great importance for many regulatory systems in the body. Potassium is involved in nerve impulses and muscle contraction, including the heart muscle. A. alnifolia also contains potassium in abundant quantity (8900 ppm). Other good sources of K and Na include tea, fruits, vegetables, and coffee. The recommended minimum daily intake of Na and K is 2.4 and 3.5 g, respectively [34].
Phosphorus macronutrient is present in bones, teeth, and numerous metabolic reactions. An average person consumes 7–10 times the amount of phosphorus recommended for an adult. Decrease in serum P is found in rickets, hyperparathyroidism, and De Toni–Fanconi syndrome. Deficiency disease or symptoms in children causes rickets, and in adults it causes osteomalacia [35]. Among the minerals found in A. alnifolia, phosphorous is the most abundant (230,000 ppm), which gives marvelous support to the pharmacological results related to the phosphorus action.
Macronutrient Ca, Mg, K, and H cation ratio are important for maintaining good health and are considered the primary structural bone minerals. Magnesium is a powerful anti-inflammatory mineral. Calcium (1800 ppm) is the key regulator of many cellular processes including cell signaling and proliferation, metabolism, muscle contraction, bone formation, and mineralization [36]. Boron helps to build and maintain healthy bones (our results show that boron content in A. alnifolia is 61 ppm).
Copper (Cu), a micronutrient, is part of red blood cells and skin pigments. In some studies, Zn deficiency is been associated with reduced insulin secretion and increased tissue resistance to insulin action [37]. Iron (Fe) presents mainly in the red blood cells as hemoglobin and plays a vital role in oxygen transport owing to its ability to exist in two redox states [38]. The highest proportion of iron shown in this screening is 1.03 mg/kg.
4.2. Mineral–antioxidant relationship
Antioxidant enzymes play an important role in scavenging the toxic intermediates of incomplete oxidation and modulate oxidative stress in disease condition [39,40]. Only when these metals are delivered in the diet in sufficient amounts can the animal body synthesize these antioxidant enzymes. By contrast, deficiency of these elements causes oxidative stress and damage to biological molecules and membranes. The dietary balance of all these nutrients (trace elements) is important in protecting tissues against free-radical damage as well as participating in immune function [41].
4.3. Analgesic study
The classification of antinociceptive drugs is usually based on their mechanism of action either on the CNS or on the peripheral nervous system [42]. Aspirin, the reference drug, offers relief from pain by suppressing the formation of pain substances in the peripheral tissues where prostaglandins and bradykinins are suggested to play an important role [43]. Flavonoids work by targeting prostaglandins, inhibiting prostaglandin synthetase and more specifically, the endoperoxidase.
Logically, acetone and methanol extracts outlined the flavonoid and tannin content in A. alnifolia leaf in our previous reports. It was noted that acetone is the best suitable solvent for flavonoid extraction [15]. Hence, the experiment to estimate the analgesic effects of A. alnifolia leaf extracts are confirmed with the animal model.
4.4. Anti-inflammatory study
Inflammation involves the innate and adaptive immune systems, and it is the normal response to infection. However, when allowed to continue unchecked, inflammation may result in autoimmune or autoinflammatory disorders, neurodegenerative diseases, or cancer [44].
Carrageenan-induced inflammation is a useful model to detect the oral action of anti-inflammatory agents [45], and egg albumin-induced paw edema in rats is also an in vivo model of inflammation that is used to screen agents for anti-inflammatory effects [46]. Prostaglandins are proinflammatory mediator characterized as hyperalgesic and potent vasodilators, and also contribute to erythema, edema, and pain. Hence, for treating inflammatory diseases, analgesic and anti-inflammatory agents are required to inhibit these mediators. The factor responsible for such anti-inflammatory properties of the extract might be the presence of organic substances/compounds such as tannin, which is capable of inhibiting the flamogen like the histamine and prostaglandin system [47]. Hence, the analgesic and anti-inflammatory effects produced by the chemical compounds in the extract may be attributed individually or collectively especially flavonoids and tannins.
4.5. Antipyretic study
Body heat regulation is not a static process but an ever-moving one that is important to normal body function. An antipyretic drug reduces fever by depressing inflammatory messages at both peripheral sites of tissue inflammation and within CNS thermoregulatory sites [48].
Drug abuse persists as a major problem that severely impacts individual and public health, locally as well as globally [49]. In particular, the synthetic agents irreversibly inhibit cyclooxygenase (COX-2) such as paracetamol and nimusulide, which have toxic effects on the various organs of the body, whereas natural COX-2 inhibitors have lower selectivity with fewer side effects—which explains why the search for herbal remedies with potent antipyretic activity has received momentum recently [50].
Therefore, antioxidant databases of a wide range of vegetables and fruit would be useful for dietary guidelines and epidemiological research [51]. The acetone extract of this plant shows a good temperature-reducing capability that matches with flavonoid quantity. Foods or food components that may have health benefits that reduce the risk of specific diseases or other health concerns [52] are called functional foods; hence, the plant A. alnifolia also comes under this category.
5. Conclusion
Many studies show that a diet rich in fruits and vegetables is good for reducing inflammation. The present study concluded that A. alnifolia has good nutritional values and acts as a better drug for proinflammatory mediator inhibition with its antinociceptive, anti-inflammatory, and antipyretic properties. Here, results are effective at the dose of 400 mg/kg of each extract; consequently, higher doses than 400 mg/kg may also provide better results. A subacute oral toxicity evaluation is needed to observe any abnormalities in animal body systems during long-term exposure, which might be helpful in determining the threshold dose. In addition to this, other pharmacological values stemming from traditional knowledge with the molecular mechanism of isolated active compounds may also elucidate our knowledge for novel drug development. Because this plant has been long used as food and as a traditional treatment for skin problems, this evaluation might help prove its potential. Moreover, this wild leafy vegetable is suitable for commercial cultivation because of the nutritional benefits it offers. Further research on effective extracts and compound isolation might be helpful for identifying the active compound from this functional food.
Acknowledgments
The first author is thankful to the DST-INSPIRE for their financial support.
Funding Statement
The first author is thankful to the DST-INSPIRE for their financial support.
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Katalinic V, Mozina SS, Generalic I, Skroza D, Ljubenkov I, Klancnik A. Phenolic profile, antioxidant capacity and antimicrobial activity of leaf extracts from six Vitis vinifera L. varieties. Int J Food Prop. 2013;16:45–60. [Google Scholar]
- 2. Moscow S, Jothivenkatachalam K. Study on mineral content of some Ayurvedic Indian medicinal plants. Int J Pharm Sci Res. 2012;3:294–9. [Google Scholar]
- 3. Gafar MK, Itodo AU. Proximate and mineral composition of hairy indigo leaves. Electronic J Environ Agric Food Chem. 2011;10:2007–18. [Google Scholar]
- 4. Ozcan M. Mineral contents of some plants used as condiments in Turkey. Food Chem. 2003;84:437–40. [Google Scholar]
- 5. Gerber M, Boutron-Ruault MC, Hercberg S, Riboli E, Scalbert A, Siess MH. Food and cancer: state of the art about the protective effect of fruits and vegetables. Bull Cancer. 2002;89:293–312. [PubMed] [Google Scholar]
- 6. Di Matteo V, Esposito E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzeimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Curr Drug Targets CNS Neurol Disord. 2003;2:95–107. doi: 10.2174/1568007033482959. [DOI] [PubMed] [Google Scholar]
- 7. Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanchez-Castillo CP, Dewey PJS, Aguirre A, Lara JJ, Vaca R, Leon de la Barra P, Ortiz M, Escamilla I, James WPT. The mineral content of Mexican fruits and vegetables. J Food Comp Anal. 1998;11:340–56. [Google Scholar]
- 9. Spacer CB, Breder CD. The neurologic basic of fever. N Engl J Med. 1994;330:1880–6. doi: 10.1056/NEJM199406303302609. [DOI] [PubMed] [Google Scholar]
- 10. Harris JD. Management of expected and unexpected opioid-related side effects. Clin J Pain. 2008;24:S8–13. doi: 10.1097/AJP.0b013e31816b58eb. [DOI] [PubMed] [Google Scholar]
- 11. Porreca F, Ossipov MH. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: mechanisms, implications, and management options. Am Acad Pain Med. 2009;10:654–62. doi: 10.1111/j.1526-4637.2009.00583.x. [DOI] [PubMed] [Google Scholar]
- 12. Yogamoorthi A, Sathya Priya E. Anti-inflammatory and analgesic property of methanolic extract of Spinifex littoreus (Burm.f.) Merr. J Environ Biol. 2006;27:271–3. [Google Scholar]
- 13.Sasi R, Rajendran A.Ethnobotany of some endemic plants of the Nilgiris, Southern western Ghats, India (NCPM/OP/090). National Conference on Phytomedicine; 4 and 5 October; Department of Botany, Bharathiar University; 2012. [Google Scholar]
- 14. Revathi P, Parimelazhagan T, Manian S. Ethnomedicinal plants and novel formulations used by Hooralis tribe in Sathyamangalam forests, Western Ghats of Tamil Nadu, India. J Med Plant Res. 2013;7:2083–97. [Google Scholar]
- 15. Revathi P, Parimelazhagan T, Manian S. Quantification of phenolic compounds, in vitro antioxidant Analysis and screening of chemical compounds using GC-MS in Acalypha alnifolia klein ex willd. — a leafy vegetable. Int J Pharm Biol Sci. 2013;4:973–86. [Google Scholar]
- 16.Sadasivam S, Manikam A. Biochemical methods. 3rd ed. Chennai, India: New Age International Publishers; 2008. [Google Scholar]
- 17. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin–phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 18. Ishida Y, Fugita T, Asai K. New detection and separation method for amino acid by high performance liquid chromatography. J Chromatogr. 1981;204:143–8. doi: 10.1016/s0021-9673(00)81650-7. [DOI] [PubMed] [Google Scholar]
- 19. Dickman SR, Bray RH. Colorimetric determination of phosphate. Ind Eng Chem Anal Ed. 1940;12:665–8. [Google Scholar]
- 20.Kalra YP. Handbook of reference methods for plant analysis. Boca Raton, FL: CRC Press; 1998. p. 300. [Google Scholar]
- 21.Jackson ML. Soil chemical analysis. Madras, India: Asia Publishing House; 1967. [Google Scholar]
- 22.Sadasivam S, Manikam A. Biochemical methods. 3rd ed. Chennai, India: New Age International Publishers; 1992. [Google Scholar]
- 23.Kulkarni SK. Handbook of experimental pharmacology. 2nd ed. New Delhi: Vallabh Prakashan; 1993. pp. 49–51. [Google Scholar]
- 24.Turner RA. Screening methods in pharmacology. In: Turner RA, Hebborn P, editors. Analgesic. New York: Academic Press; 1965. pp. 100–2. [Google Scholar]
- 25. Gupta RK, Tandon VR. Antinociceptive activity of Vitex negundo Linn. leaf extract. Indian J Physiol Pharmacol. 2005;49:163–70. [PubMed] [Google Scholar]
- 26. Winter CA, Portar CC. Effect of alterations in side chain upon anti-inflammatory and liver glycogen activities of hydrocortisone esters. J Am Pharm Sci Educ. 1957;46:515–9. doi: 10.1002/jps.3030460902. [DOI] [PubMed] [Google Scholar]
- 27. Weithmann KU, Jeske S, Schlotte V. Effect of Leflunomide on constitutive and inducible pathways of cellular eicosanoid generation. Agent Actions. 1994;41:164–70. doi: 10.1007/BF02001911. [DOI] [PubMed] [Google Scholar]
- 28.Hess SM, Millonig RC. Assays for anti-inflammatory agents. In: Lepow IH, Ward PA, editors. Inflammation mechanisms and control. London: Academic Press; 1972. pp. 1–12. [Google Scholar]
- 29. Olajide OA, Awe SO, Makinde JM, Ekhelar AI, Olusola A, Morebise O, Okpako DT. Studies on the anti-inflammatory, antipyretic and analgesic properties of Alstonia boonei stem bark. J Ethnopharmacol. 2000;71(1–2):179–86. doi: 10.1016/s0378-8741(99)00200-7. [DOI] [PubMed] [Google Scholar]
- 30. Jain BB, Rathi BS, Thakurdesai PA, Bodhankar SL. Antipyretic activity of aqueous extract of leaves of Cocculus hirsutus. Ind J Nat Prod. 2007;23:26–9. [Google Scholar]
- 31.Renter E. Magnesium is a powerful natural anti-inflammatory mineral. Natural Society; 2013. http://naturalsociety.com/magnesium-is-a-powerful-natural-anti-inflammatory-mineral/#ixzz2n0GDXQm3 . [Google Scholar]
- 32. Soetan KO, Olaiya CO, Oyewole OE. The importance of mineral elements for humans, domestic animals and plants — a review. Afr J Food Sci. 2010;4:200–2. [Google Scholar]
- 33.Baysal A. Fundamentals of nutrition. Ankara: Hatipoğlu Press; 2002. p. 14. [in Turkish] [Google Scholar]
- 34. Streeten DHP, Williams EMV. Loss of cellular potassium as a cause of intestinal paralysis in dogs. J Physiol. 1952;118:149–70. doi: 10.1113/jphysiol.1952.sp004782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray RK, Granner DK, Mayes PA, Rodwell VW. Harper’s biochemistry. 25th ed. USA: McGraw-Hill Health Profession Division; 2000. [Google Scholar]
- 36. Abdulkadir IE, Aliyu AB, Ibrahim MA, Audu SBD, Oyewale AO. Antioxidant activity and mineral elements profiles of Isoberlinia Doka leaves from Nigeria. Aust J Basic Appl Sci. 2011;5:2507–12. [Google Scholar]
- 37.Kirchgessner M, Roth HP, Weigand E. Biochemical changes in Zinc deficiency. In: Prasad AS, Oberleas D, editors. Trace elements in human health and disease: zinc and copper. Vol. 1. New York: Academic Press; 1976. pp. 189–266. [Google Scholar]
- 38. Minihane AM, Rimbach G. Iron absorption and the iron binding and anti-oxidant properties of phytic acid. Int J Food Sci Technol. 2002;37:741–8. [Google Scholar]
- 39. Weiss WP, Hogan JS, Todhunter DA, Smith KL. Effect of vitamin E supplementation in diets with a low concentration of selenium on mammary gland health of dairy cows. J Dairy Sci. 1997;80:1728. doi: 10.3168/jds.S0022-0302(97)76105-8. [DOI] [PubMed] [Google Scholar]
- 40. Kandasamy N, Ashokkumar N. Myricetin modulates streptozotocinec admium induced oxidative stress in long term experimental diabetic nephrotoxic rats. J Funct Foods. 2013;5(3):1466–77. [Google Scholar]
- 41.Mcdowell LR, Wilkinson N, Madison R, Felix T.Vitamins and minerals functioning as antioxidants with supplementation considerations. Florida Ruminant Nutrition Symposium, Best Western Gateway Grand; Gainesville, FL; 2007. [Google Scholar]
- 42. Planas E, Sanchez S, Rodriguez L, Pol O, Puig MM. Antinociceptive, anti-edema effects of liposomal morphine during acute inflammation of the rat paw. Pharmacology. 2000;60:121–7. doi: 10.1159/000028356. [DOI] [PubMed] [Google Scholar]
- 43. Drabu S, Chaturvedi S, Sharma M. Analgesic activity of methanolic extract from the fruits of Trichosanthus dioica. Int J Res Pharm Biomed Sci. 2012;3:399–405. [Google Scholar]
- 44. Dinarello CA. Anti-inflammatory agents: present and future cell. Cell. 2010;140:935–50. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Di Rosa M, Giround JP, Willoughby DA. Studies of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971;104:15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- 46. Amos S, Chindo B, Edmond I, Akah P, Wambebe C, Gamaniel K. Anti-inflammatory and antinociceptive effects of Ficus Platyphylla in rats and mice. J Herbs Spices Med Plants. 2002;9:47–53. [Google Scholar]
- 47. Muruganandham S, Pant S, Srinivasan K, et al. Anti inflammatory activity of Syzygium cumini bark. Fitoterapia. 1999;72:369–75. doi: 10.1016/s0367-326x(00)00325-7. [DOI] [PubMed] [Google Scholar]
- 48. Aronoff MD, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111:304–15. doi: 10.1016/s0002-9343(01)00834-8. [DOI] [PubMed] [Google Scholar]
- 49. Chiu WT. Special Issue of the International Conference on Global Health. J Food Drug Anal. 2013:1. [Google Scholar]
- 50.Guyton AC, Hall JE. Textbook of medical physiology. 9th ed. Philadelphia: W.B. Saunders Company; 1998. pp. 920–2. [Google Scholar]
- 51. Deng GF, Lin X, Xu XR, Gao LL, Xie JF, Li HB. Antioxidant capacities and total phenolic contents of 56 vegetables. J Funct Foods. 2013;5(1):260–6. [Google Scholar]
- 52. National Institute of Nutrition. Consumer awareness of and attitudes towards functional foods, highlights and implications for informing consumer. Leaflet. 2000:4. [Google Scholar]