Fig. 8 |. Single-atom insertions that leverage carbonyl chemistry.
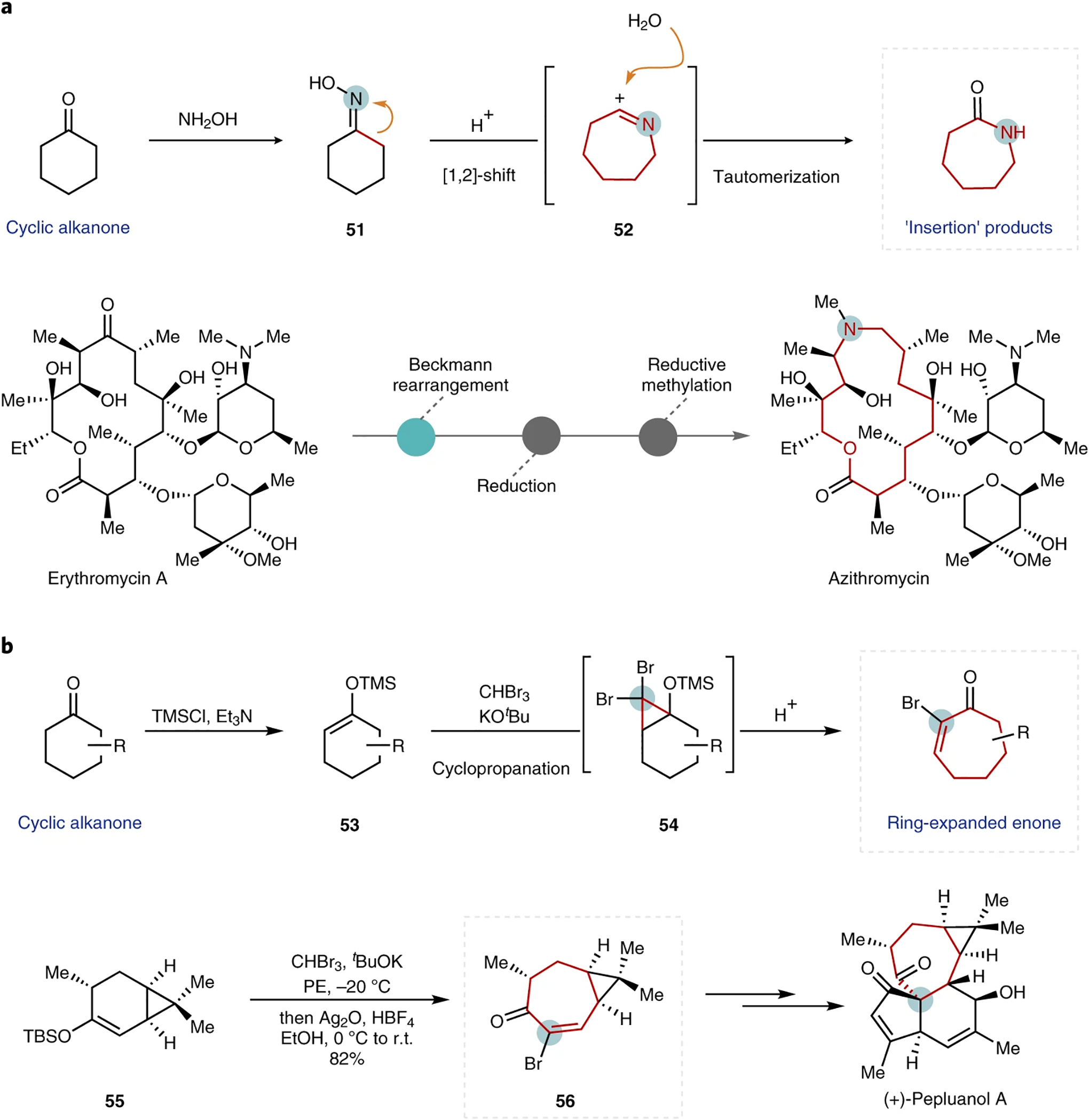
a, General reaction mechanism for a Beckmann rearrangement reaction (top) with the application demonstrated in the synthesis of azithromycin66 (bottom). b, Representative example of a cyclopropanation reaction used to achieve ring expansion (top) and a synthetic application shown in the synthesis of (+)-pepluanol A72 (bottom). Inserted atoms are circled, and expanded rings are highlighted in red for clarity. TMS, trimethylsilyl; TBS, tert-butyldimethylsilyl.
