Abstract
Background
Previous studies suggest the association between early-life weight gain and asthma. It remains unclear whether early-life weight gain is associated with atopic or non-atopic asthma. This study aimed to investigate whether early-life weight gain is associated with atopic or non-atopic asthma.
Methods
Included in this study were 1343 singleton-birth children (761 boys, 57%) born between January 2010 and December 2011 participating in the Longitudinal Investigation of Global Health in Taiwanese Schoolchildren (LIGHTS) cohort were evaluated by a modified International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire and interviewed by pediatricians between July 1, 2016 and May 31, 2018 at the mean age of 6.4 years. Weight gain z-scores during the first 6, 12, and 18 months of life were classified into 4 groups: slow (below −0.67), on track (−0.67 to 0.67), rapid (0.67 to 1.28), and extremely rapid (above 1.28). The main outcomes were atopic and non-atopic asthma. Asthma was defined as having physician-diagnosed asthma and the presence of wheeze or asthma exacerbations in the last 12 months. Atopy was determined by Phadiatop Infant.
Results
The extremely rapid weight gain group of children during the first 6, 12, and 18 months of life was significantly associated with an increased risk of non-atopic asthma (adjusted odd ratio [AOR], 2.14, 95% confidence interval [CI], 1.01–4.53 for the first 6 months; AOR, 2.86, 95% CI, 1.34–6.14 for the first 12 months; AOR, 3.26, 95% CI 1.49–7.15 for the first 18 months) compared with the on track group. No significant association was found in atopic asthma. A sex-stratified analysis revealed the association of early-life weight gain with non-atopic asthma was statistically significant only in boys (AOR, 4.24, 95% CI, 1.44–12.50).
Conclusion
Extremely rapid weight gain during the first 6–18 months of life was significantly associated with 2.1- to 3.3-fold increased risk of non-atopic asthma, with a more pronounced risk found in boys.
Keywords: Weight gain, Asthma, Atopy, Children, Cohort study
Introduction
Asthma is the most common chronic respiratory disease in children and poses a significant burden to the affected children as well as healthcare systems.1,2 Asthma prevalence worldwide has been increasing in recent decades based on the data from the International Study of Asthma and Allergies in Childhood (ISAAC), an international multicenter epidemiological study.3 In parallel, childhood obesity has also increased in recent decades.4
Growing evidence supports that obesity is an important risk factor for asthma development.5, 6, 7, 8, 9 Since childhood weight gain is a dynamic process, the temporal sequence might better reflect the time window of gaining excess weight and the subsequent development of asthma. Some studies have explored the associations between growth patterns in early life and asthma.10, 11, 12, 13, 14, 15 For example, a meta-analysis including 147,252 European children in 31 birth cohort studies found that rapid weight gain in infancy was positively associated with childhood asthma outcomes.10 In a birth cohort in the United States, Tsai et al reported that children with extremely rapid weight gain at the first 4, 12, and 24 months of life were each associated with an increased risk of asthma in childhood compared with the on track group of children.11
Asthma is a heterogeneous disease consisting of several phenotypes. Asthma can be classified as atopic or non-atopic, depending on the presence or absence of atopy based on a positive skin prick test or specific immunoglobulin E (IgE) antibodies against common allergens.16,17 Although atopic children are more likely to have asthma, a considerable proportion of children with asthma do not appear to be atopic.18 Despite similar clinical features between atopic and non-atopic asthma, the risk factors and underlying immunological mechanisms are probably different.19 However, several studies have ignored the distinction between atopic and non-atopic asthma even though these phenotypes may have distinct causal mechanisms.
Although previous studies have reported the association of early-life growth patterns with childhood asthma,10, 11, 12, 13, 14, 15 it remains unclear whether early-life excess weight gain is associated with atopic or non-atopic asthma. In the present study, we aimed to investigate the temporal association of early-life weight gain with atopic or non-atopic asthma among Asian children in the Longitudinal Investigation of Global Health in Taiwanese Schoolchildren (LIGHTS) cohort.
Methods
Study design
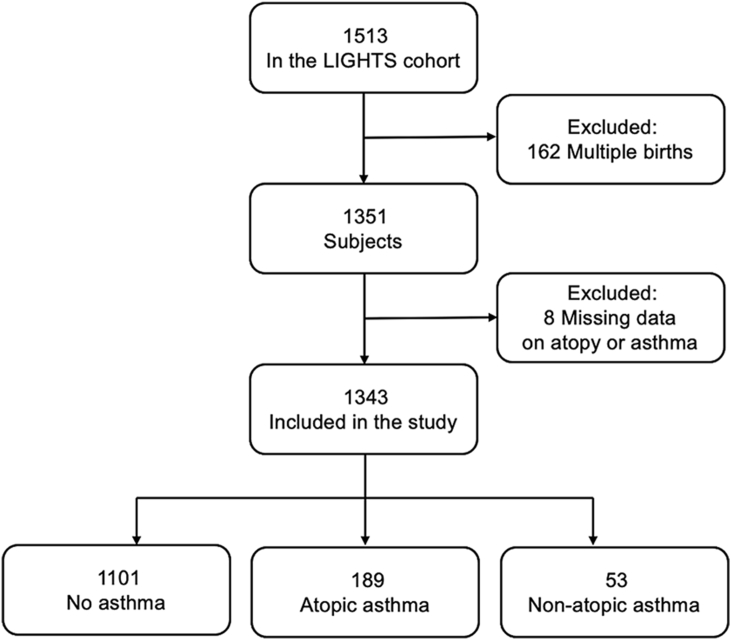
This study included 1343 singleton-birth children participating in the LIGHTS study. The LIGHTS population-based cohort consists of 1513 children who were born in 2010 and 2011 and attended well-child visits and follow-up visits in the Chang Gung Memorial Hospital (CGMH).20, 21, 22 After excluding multiple births (n = 162) and missing data on asthma or atopy (n = 8), 1343 children were included in the present study. The flow chart for the enrollment process of study subjects is shown in Fig. 1. The perinatal health information was obtained from electronic medical records in the CGMH. A modified ISAAC questionnaire was applied to collect demographic and epidemiologic data, general health information, and clinical data from parents of study children. All participants had an interview conducted by pediatricians. Blood samples were drawn for subsequent measurement of serum allergen-specific IgE. The study was approved by the Institutional Review Board of Chang Gung Medical Foundation (No. 201600334A3). Written informed consent was provided by the parent or legal guardian of each participant.
Fig. 1.
Flow chart for the enrollment process of study subjects. Abbreviation: LIGHTS: Longitudinal Investigation of Global Health in Taiwanese Schoolchildren.
Weight gain measurement
Child's weight and height at the age of 6, 12, and 18 months, were measured by trained pediatric nurses at well-child visits based on standard clinical procedures. Specifically, older children were measured without shoes and wearing only a t-shirt and underwear, and for infants only a diaper was allowed. This study defined change in weight gain z-scores during 3 different time periods: from birth to the first 6, 12, and 18 months. For each time period, we calculated weight-for-age z-scores, using the Stata program for Child Growth Standards developed by the World Health Organization (WHO),23 and the change in weight-for-age z-scores during the corresponding period. We further classified change in weight gain z-scores into 4 groups: (1) slow weight gain, defined as weight gain z-score below −0.67; (2) on track, defined as weight gain z-score between −0.67 and 0.67; (3) rapid weight gain, defined as weight gain z-score between 0.67 and 1.28; and (4) extremely rapid weight gain, defined as weight gain z-score above 1.28, respectively. The numbers of children in the 4 weight gain groups during the first 6, 12, and 18 months are shown in Table S1.
Outcome assessment
The primary outcomes were atopic asthma and non-atopic asthma, which were evaluated during the follow-up visit at the mean age of 6.4 years. Asthma was defined as having physician-diagnosed asthma (either by a parent-reported questionnaire or an interview by pediatricians) and the presence of wheeze or asthma exacerbations in the last 12 months. Atopy, as the secondary outcome, was defined based on the Phadiatop Infant test result (≧0.35 PAU/L) (Phadia, Uppsala, Sweden), measuring IgE against the following specific allergens: house dust mite, cat, dog, birch, timothy, ragweed, wall pellitory, egg white, cow's milk, peanut, and shrimp.24 Asthma was further divided into atopic and non-atopic asthma based on presence or absence of atopy.
Statistical analysis
For demographic characteristics, categorical variables were compared and tested using chi-squared test; and continuous variables were tested using analysis of variance (ANOVA). Early-life weight gain was treated as a categorical variable with 4 groups (slow weight gain, on track [reference group], rapid weight gain, and extremely rapid weight gain). Multiple logistic regression analyses were applied to examine the associations of early-life weight gain group with atopic asthma, non-atopic asthma, and atopy, separately, with adjustment of relevant covariates. The adjusted covariates were listed as follows: child's age, sex, current body mass index (BMI), prematurity (based on gestational age [GA]: term [≥37 weeks] and preterm [<37 weeks]), birth order, breastfeeding (<3 months and ≥3 months), maternal asthma, maternal age at delivery, maternal pre-pregnancy BMI, gestational diabetes, maternal smoking, maternal education, and household income. To evaluate potential effect modification, we performed subgroup analysis stratified by child's sex. We declare a P-value less than 0.05 as statistical significance. Statistical analyses were performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA).
Results
Characteristics of the study participants
Table 1 presents the characteristics of the 1343 study subjects (761 boys, 57%; mean age, 6.4 years). Among the 1343 study children, 189 (14.1%) and 53 (4.0%) had atopic asthma and non-atopic asthma, respectively. The prevalence of atopy was 65.0% among the study population in this study. Children with atopic asthma and non-atopic asthma both tended to have a higher proportion of maternal asthma than those without asthma. Clinical characteristics of children in the 4 weight gain groups are shown in Table S2.
Table 1.
Characteristics of study subjects.
| Variable | No asthma (n = 1101) | Atopic asthma (n = 189) | Non-atopic asthma (n = 53) |
|---|---|---|---|
| Age, year, mean ± SD | 6.4 ± 0.4 | 6.4 ± 0.4 | 6.5 ± 0.4 |
| Sex, male, n (%) | 615 (56.0) | 118 (62.4) | 28 (52.8) |
| BMI, kg/m2, mean ± SD | 15.8 ± 2.4 | 16.1 ± 2.4 | 15.4 ± 1.8 |
| Prematurity, n (%)a | 167 (15.2) | 28 (14.8) | 9 (17.0) |
| Birth order, n (%)b | |||
| First | 605 (55.0) | 113 (59.8) | 32 (60.4) |
| Second | 394 (35.8) | 68 (36.0) | 17 (32.1) |
| Third or later | 102 (9.2) | 8 (4.2) | 4 (7.5) |
| Breastfeeding, n (%) | |||
| <3 months | 546 (49.9) | 96 (50.8) | 22 (41.5) |
| ≥3 months | 552 (50.1) | 93 (49.2) | 31 (58.5) |
| Maternal asthma, n (%)b | 56 (5.1) | 21 (11.2) | 7 (13.5) |
| Maternal age at delivery, year, mean ± SD | 32.2 ± 4.1 | 31.9 ± 4.1 | 32.4 ± 3.9 |
| Maternal pre-pregnancy BMI, kg/m2 | 21.2 ± 3.3 | 21.0 ± 3.0 | 21.3 ± 3.5 |
| Gestational diabetes, n (%) | 58 (5.3) | 11 (5.8) | 4 (7.5) |
| Maternal smoking, n (%) | 39 (3.5) | 6 (3.2) | 0 (0.0) |
| Maternal university education, n (%) | 875 (79.8) | 152 (80.4) | 46 (86.8) |
| Annual household income, n (%) | |||
| <300,000 NTD | 40 (3.7) | 4 (2.1) | 1 (1.9) |
| 300,000–600,000 NTD | 176 (16.1) | 30 (16.0) | 14 (26.4) |
| 600,000–900,000 NTD | 243 (22.3) | 38 (20.2) | 8 (15.1) |
| 900,000–1,200,000 NTD | 300 (27.5) | 56 (29.8) | 15 (28.3) |
| >1,200,000 NTD | 333 (30.5) | 60 (31.9) | 15 (28.3) |
Abbreviation: SD, standard deviation; BMI, body mass index; NTD, new Taiwan dollar.
Classified based on gestational age: term (≧37 weeks) and preterm (<37 weeks).
P < 0.05 across three groups
Association of early-life weight gain with atopic and non-atopic asthma
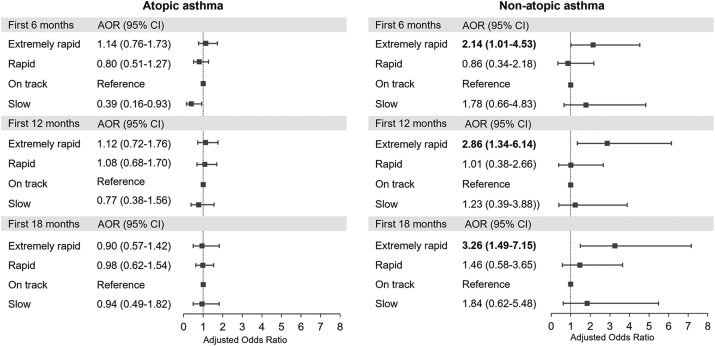
Fig. 2 shows that the extremely rapid weight gain group during the first 6, 12, and 18 months of life was significantly associated with an increased risk of non-atopic asthma (adjusted odds ratio (AOR), 2.14; 95% confidence interval (CI), 1.01–4.53 for the first 6 months; AOR, 2.86; 95% CI, 1.34–6.14 for the first 12 months; AOR, 3.26; 95% CI, 1.49–7.15 for the first 18 months) compared with the on track group, after adjustment for child's age, sex, current BMI, prematurity, birth order, breastfeeding, maternal asthma, maternal age at delivery, maternal pre-pregnancy BMI, gestational diabetes, maternal smoking, maternal education, and household income. In contrast, no significant relationship was found between weight gain groups during the first 6, 12, and 18 months and atopic asthma (Fig. 2). A sensitivity analysis was performed by excluding children who were born preterm. The increased risk of non-atopic asthma among the extremely rapid weight gain group remained significant after excluding children born preterm (Figure S1).
Fig. 2.
Associations of weight gain in the first 6, 12, and 18 months with atopic and non-atopic asthma∗. ∗Adjusted for child's age, sex, current body mass index, prematurity, birth order, breastfeeding, maternal asthma, maternal age at delivery, maternal pre-pregnancy body mass index, gestational diabetes, maternal smoking, maternal education, and household income. Bold values denote statistical significance at P < 0.05. Abbreviations: AOR, adjusted odds ratio; CI, confidence interval.
Association of early-life weight gain with atopy
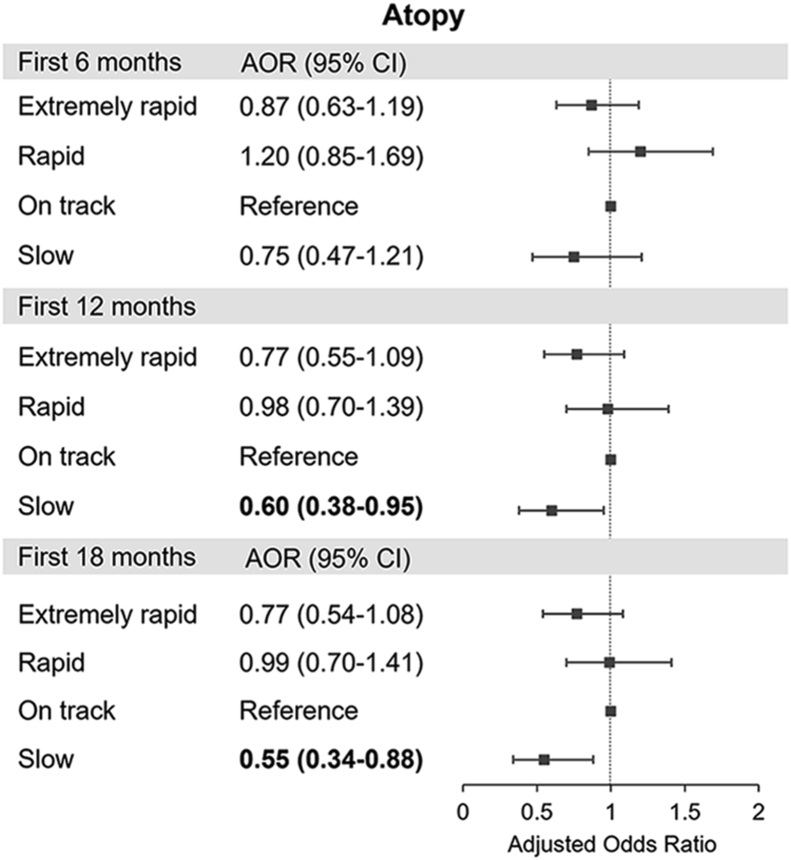
There was no significant association of extremely rapid and rapid weight gain groups during the first 6, 12, and 18 months with atopy, although the slow weight gain group during the first 12 and 18 months of life was associated with a marginally significant decreased risk of atopy (AOR, 0.60; 95% CI, 0.38–0.95 for the first 12 months; AOR, 0.55; 95% CI, 0.34–0.88 for the first 18 months) (Fig. 3).
Fig. 3.
Associations of weight gain in the first 6, 12, and 18 months with atopy∗. ∗Adjusted for child's age, sex, current body mass index, prematurity, birth order, breastfeeding, maternal asthma, maternal age at delivery, maternal pre-pregnancy body mass index, gestational diabetes, maternal smoking, maternal education, and household income. Abbreviations: AOR, adjusted odds ratio; CI, confidence interval.
Association of early-life weight gain with non-atopic asthma stratified by child's sex
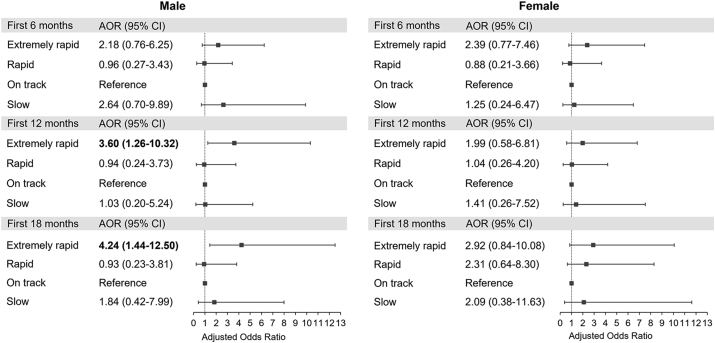
We further performed a stratified analysis to evaluate whether the detrimental effect of weight gain in early life on non-atopic asthma differed by child's sex. Fig. 4 shows significant association of extremely rapid weight gain during the first 12 and 18 months of life with non-atopic asthma among boys (AOR, 3.60; 95% CI, 1.26–10.32 for the first 12 months; AOR, 4.24; 95% CI, 1.44–12.50 for the first 18 months), but not among girls.
Fig. 4.
Associations of weight gain in the first 6, 12, and 18 months with non-atopic asthma, stratified by sex∗. ∗Adjusted for child's age, sex, current body mass index, prematurity, birth order, breastfeeding, maternal asthma, maternal age at delivery, maternal pre-pregnancy body mass index, gestational diabetes, maternal smoking, maternal education, and household income. Bold values denote statistical significance at P < 0.05. Abbreviations: AOR, adjusted odds ratio; CI, confidence interval.
Discussion
To our knowledge, this is the first cohort study of Asian children to investigate the temporal association of early-life weight gain with atopic or non-atopic asthma. This study of 1343 singleton-birth Asian children demonstrates that extremely rapid weight gain during the first 6, 12, and 18 months of life were all significantly associated with an increased risk of non-atopic asthma (AORs ranging from 2.14 to 3.26), but not atopic asthma. The results remain significant after adjustment for a range of factors, including child's age, sex, current BMI, prematurity, birth order, breastfeeding, maternal asthma, maternal age at delivery, maternal pre-pregnancy BMI, gestational diabetes, maternal smoking, maternal education, and household income. This study provides new evidence that the detrimental effect of early-life weight gain on the subsequent development of childhood asthma is mainly confined to non-atopic asthma and the effect is probably more pronounced among boys than girls.
While several previous studies reported the association of excess weight gain in early life with childhood asthma,10, 11, 12, 13, 14, 15 the mechanism remains unknown. Our findings imply that the effect of excess weight gain in early life on the subsequent development of childhood asthma is likely mediated by non-IgE-mediated mechanisms. In addition, the increased risk of non-atopic asthma was found in the extremely rapid weight gain group, but not in the rapid weight gain group, suggesting non-linear threshold relations of early-life weight gain with the risk of non-atopic asthma. Rapid weight gain in early life has been recognized as an important determinant of childhood obesity.25, 26, 27 In addition, previous studies suggested a stronger association of obesity with asthma in non-atopic children than in atopic children.28,29 In a National Health and Nutrition Examination Survey among 16,074 US children and adolescents aged 2–19 years, Visness and colleagues reported that the association of obesity with asthma was stronger in non-atopic children than in atopic children.28 Another national sample of US children also found that children with non-atopic asthma (but not atopic asthma) had higher BMI than non-asthmatic children.29 The relationship between obesity and non-atopic asthma was also suggested in adults.30,31 A study of 4060 Australian adults showed that central obesity was associated with non-atopic asthma but not atopic asthma.30 A recent cross-trait genome-wide association study of 457,822 European adults demonstrated a positive genetic correlation of obesity with non-atopic asthma but not with atopic asthma.31 Accordingly, these data may indirectly support our findings and suggest that excess weight gain in early life increases the risk of subsequent obesity and further increases the risk of developing non-atopic asthma.
Our findings are in line with studies reported by other groups that found no association of rapid weight gain with atopy.32, 33, 34 For example, the results from a cohort study in the United Kingdom revealed that postnatal growth and adiposity gain did not influence the relative risk of atopy in 1548 full-term infants followed from birth to 3 years.32 A large trial in the Republic of Belarus with 12,171 term infants showed no evidence of consistent associations between weight gain from infancy to mid-childhood and atopic status at the age of 6.5 years.33 These findings, taken together, support our speculation that the relationship between excess weight gain in early life and asthma may be mediated through an atopy-independent pathway.
Although sex difference in the relationship between childhood obesity and asthma has been reported by other studies,35, 36, 37 there is scant evidence for sex difference on the association of early-life weight gain with asthma. In a US birth cohort study, Tsai et al reported no sex difference in the association between early-life weight gain and asthma.11 Our results suggest that boys are more sensitive to the detrimental effect of extremely rapid weight gain on non-atopic asthma than girls. However, the mechanisms underlying the sex difference remain uncovered. Further studies are needed to investigate the role and mechanism of sex in the association between early-life weight gain and asthma.
The present study has several strengths. First, the study was conducted in a large population-based cohort of Asian children with comprehensive measurement of serum allergen-specific IgE as an objective marker of allergic sensitization. Second, the information of maternal and perinatal data was obtained directly from medical records instead of self-reports, avoiding potential recall bias. Some potential limitations should also be noted. First, the study cohort only included an Asian pediatric population. Whether the results can be generalized to other ethnic populations remains to be confirmed. Second, although several pertinent factors have been controlled and adjusted in the analyses, it remains possible that the observed increased risk might be partially explained by unmeasured confounding factors. Third, the small sample size for the non-atopic asthma group has resulted in the wide 95% confidence intervals. However, the effect size increases as the duration of rapid weight gain increases suggesting the extreme phenotype may be related biologically to asthma risk in this way. Further validation studies with larger sample sizes are needed.
Conclusion
This population-based cohort study demonstrates that extremely rapid weight gain during the first 6, 12, and 18 months of life poses an increased risk of subsequently developing non-atopic asthma, but not atopic asthma, by the age of 6 years in Asian children. The association is probably more pronounced among boys than girls. Public awareness and effective strategies in avoiding excess weight gain in early life may be helpful in preventing the development of non-atopic asthma in childhood.
Abbreviations
ISAAC, International Study of Asthma and Allergies in Childhood; IgE, immunoglobulin E; LIGHTS, Longitudinal Investigation of Global Health in Taiwanese Schoolchildren; CGMH, Chang Gung Memorial Hospital; ANOVA, analysis of variance; BMI, body mass index; GA, gestational age; AOR, adjusted odds ratio; CI, confidence interval; SD, standard deviation; NTD, new Taiwan dollar.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, T-C. Yao, upon reasonable request.
Author contributions
C-H. Ho conceptualized and designed the study, carried the data analyses and interpretation of the data, and drafted manuscript. C-C. Gau, W-F. Lee, H. Fang, C-H. Lin, C-H. Chu, Y-S. Huang, Y-W. Huang assisted in participant recruitment, cohort maintenance, and acquisition of data. H-Y. Huang assisted in statistical analysis. H-J. Tsai assisted in data analysis and interpretation of the data, and critically revised manuscript. T-C. Yao conceptualized and designed the study, coordinated data collection and statistical analyses, drafted and revised the manuscript, and raised funding, and supervised the study. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Ethics statement
The study was approved by the Institutional Review Board of Chang Gung Medical Foundation (No. 201600334A3). Written informed consent was provided by the parent or legal guardian of each participant. The procedures followed in this study were in accordance with the ethical standards of The Institutional Review Boards of Chang Gung Memorial Hospital and with the Helsinki Declaration of 1975, as revised in 1983.
Author’s consent for publication
We give our consent for the publication of this manuscript to be published in the World Allergy Organization Journal, if it is accepted for publication.
Declaration of competing interest
The authors report no competing interests.
Submission declaration
We confirm that this manuscript has not been submitted or is not simultaneously being submitted elsewhere, and that no portion of the data has been or will be published in proceedings or transactions of meetings or symposium volumes.
Acknowledgements
Members of the LIGHTS study group are: Tsung-Chieh Yao (Principle Investigator), Hui-Ju Tsai (Co-Principle Investigator), Chao-Yi Wu, Hung-Yi Lu, Chun-Chun Gau, Wan-Fang Lee, Li-Lun Lin, Shu-Jung Huang, Yu-Tung Lan, Chung-Chieh Hung, I-Chun Lu, Fang-Yu Chang, Chun-Hui Chu, Chi-Yen Hung, Kun-Lin Lu, Yu-Wen Huang, Yin-Shan Huang, Ching-Hua Lin, Hsin Fang, Zhao-Ting Tsai, Po-Hsiang Kao, Cha-Shien Yen, Yen-Ju Shen, Chi-Wei Chiu, Tzu-Hsiang Weng, Chia-Hua Ho, Chi-Wen Huang, Yu-Tang Juan, Ju Chang-Chien, Hsin-Yi Huang, Yu-Lung Tseng, Wan-Chin Lin, and Shih-Ling Wang. The authors thank the study participants and their parents for their active participation in the study. This work was supported by the Ministry of Science and Technology, Taiwan (MOST 109-2314-B-182-042-MY3; MOST 106-2314-B-182-051-MY3; PI: Tsai, MOST 107-2314-B-400-031-MY3) and Chang Gung Medical Foundation, Taiwan (CMRPG3F1711∼3, CMRPG3K1371∼2, CMRPG3J0121∼3, CMRPG3J0161∼3, and CORPG3F0361).
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2022.100672.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Asher I., Pearce N. Global burden of asthma among children. Int J Tubercul Lung Dis. Nov 2014;18(11):1269–1278. doi: 10.5588/ijtld.14.0170. [DOI] [PubMed] [Google Scholar]
- 2.To T., Dell S., Dick P., Cicutto L. The burden of illness experienced by young children associated with asthma: a population-based cohort study. J Asthma. Jan-Feb 2008;45(1):45–49. doi: 10.1080/02770900701815743. [DOI] [PubMed] [Google Scholar]
- 3.Ellwood P., Asher M.I., Beasley R., Clayton T.O., Stewart A.W. The international study of asthma and allergies in childhood (ISAAC): phase three rationale and methods. Int J Tubercul Lung Dis. Jan 2005;9(1):10–16. [PubMed] [Google Scholar]
- 4.Di Cesare M., Sorić M., Bovet P., et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. Nov 25 2019;17(1):212. doi: 10.1186/s12916-019-1449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters U., Dixon A.E., Forno E. Obesity and asthma. J Allergy Clin Immunol. Apr 2018;141(4):1169–1179. doi: 10.1016/j.jaci.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sansone F., Attanasi M., Di Pillo S., Chiarelli F. Asthma and obesity in children. Biomedicines. Jul 21 2020;8(7) doi: 10.3390/biomedicines8070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng X., Ma J., Yuan Y., Zhang Z., Niu W. Association between overweight or obesity and the risk for childhood asthma and wheeze: an updated meta-analysis on 18 articles and 73 252 children. Pediatr Obes. Sep 2019;14(9) doi: 10.1111/ijpo.12532. [DOI] [PubMed] [Google Scholar]
- 8.Beuther D.A., Sutherland E.R. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. Apr 1 2007;175(7):661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao T.C., Ou L.S., Yeh K.W., Lee W.I., Chen L.C., Huang J.L. Associations of age, gender, and BMI with prevalence of allergic diseases in children: PATCH study. J Asthma. Jun 2011;48(5):503–510. doi: 10.3109/02770903.2011.576743. [DOI] [PubMed] [Google Scholar]
- 10.Sonnenschein-van der Voort A.M., Arends L.R., de Jongste J.C., et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol. May 2014;133(5):1317–1329. doi: 10.1016/j.jaci.2013.12.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai H.J., Wang G., Hong X., et al. Early life weight gain and development of childhood asthma in a prospective birth cohort. Ann Am Thorac Soc. Oct 2018;15(10):1197–1204. doi: 10.1513/AnnalsATS.201712-921OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rzehak P., Wijga A.H., Keil T., et al. Body mass index trajectory classes and incident asthma in childhood: results from 8 European Birth Cohorts--a Global Allergy and Asthma European Network initiative. J Allergy Clin Immunol. Jun 2013;131(6):1528–1536. doi: 10.1016/j.jaci.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Flexeder C., Thiering E., Bruske I., et al. Growth velocity during infancy and onset of asthma in school-aged children. Allergy. Feb 2012;67(2):257–264. doi: 10.1111/j.1398-9995.2011.02748.x. [DOI] [PubMed] [Google Scholar]
- 14.van der Gugten A.C., Koopman M., Evelein A.M., Verheij T.J., Uiterwaal C.S., van der Ent C.K. Rapid early weight gain is associated with wheeze and reduced lung function in childhood. Eur Respir J. Feb 2012;39(2):403–410. doi: 10.1183/09031936.00188310. [DOI] [PubMed] [Google Scholar]
- 15.Paul I.M., Camera L., Zeiger R.S., et al. Relationship between infant weight gain and later asthma. Pediatr Allergy Immunol. Feb 2010;21(1 Pt 1):82–89. doi: 10.1111/j.1399-3038.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawson J.A., Chu L.M., Rennie D.C., et al. Prevalence, risk factors, and clinical outcomes of atopic and nonatopic asthma among rural children. Ann Allergy Asthma Immunol. Mar 2017;118(3):304–310. doi: 10.1016/j.anai.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Moncayo A.L., Vaca M., Oviedo G., et al. Risk factors for atopic and non-atopic asthma in a rural area of Ecuador. Thorax. May 2010;65(5):409–416. doi: 10.1136/thx.2009.126490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strina A., Barreto M.L., Cooper P.J., Rodrigues L.C. Risk factors for non-atopic asthma/wheeze in children and adolescents: a systematic review. Emerg Themes Epidemiol. 2014;11:5. doi: 10.1186/1742-7622-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinisgalli S., Collins M.S., Schramm C.M. Clinical features cannot distinguish allergic from non-allergic asthma in children. J Asthma. Feb 2012;49(1):51–56. doi: 10.3109/02770903.2011.631244. [DOI] [PubMed] [Google Scholar]
- 20.Lu H.Y., Chiu C.W., Kao P.H., et al. Association between maternal age at delivery and allergic rhinitis in schoolchildren: a population-based study. World Allergy Organ J. Jun 2020;13(6) doi: 10.1016/j.waojou.2020.100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao T.C., Huang H.Y., Pan W.C., et al. Association of prenatal exposure to fine particulate matter pollution with childhood eczema. Allergy. Jul 2021;76(7):2241–2245. doi: 10.1111/all.14738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang-Chien J., Huang H.Y., Tsai H.J., et al. Metabolomic differences of exhaled breath condensate among children with and without asthma. Pediatr Allergy Immunol. Feb 2021;32(2):264–272. doi: 10.1111/pai.13368. [DOI] [PubMed] [Google Scholar]
- 23.WHO. WHO Child Growth Standards: Length/height-For-Age, Weight-For-Age, Weight-For-Length, Weight-For-Height and Body Mass Index-For-Age: Methods and Development. https://www.who.int/publications/i/item/924154693X.
- 24.Ballardini N., Nilsson C., Nilsson M., Lilja G. ImmunoCAP Phadiatop Infant--a new blood test for detecting IgE sensitisation in children at 2 years of age. Allergy. Mar 2006;61(3):337–343. doi: 10.1111/j.1398-9995.2005.00936.x. [DOI] [PubMed] [Google Scholar]
- 25.Li Y.F., Lin S.J., Chiang T.L. Timing of rapid weight gain and its effect on subsequent overweight or obesity in childhood: findings from a longitudinal birth cohort study. BMC Pediatr. Jun 12 2020;20(1):293. doi: 10.1186/s12887-020-02184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ong K.K., Loos R.J. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. Aug 2006;95(8):904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 27.Ong K.K., Ahmed M.L., Emmett P.M., Preece M.A., Dunger D.B. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. Br Med J. Apr 8 2000;320(7240):967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Visness C.M., London S.J., Daniels J.L., et al. Association of childhood obesity with atopic and nonatopic asthma: results from the National Health and Nutrition Examination Survey 1999-2006. J Asthma. Sep 2010;47(7):822–829. doi: 10.3109/02770903.2010.489388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelley C.F., Mannino D.M., Homa D.M., Savage-Brown A., Holguin F. Asthma phenotypes, risk factors, and measures of severity in a national sample of US children. Pediatrics. Mar 2005;115(3):726–731. doi: 10.1542/peds.2004-0529. 115/3/726 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Appleton S.L., Adams R.J., Wilson D.H., Taylor A.W., Ruffin R.E. Central obesity is associated with nonatopic but not atopic asthma in a representative population sample. J Allergy Clin Immunol. Dec 2006;118(6):1284–1291. doi: 10.1016/j.jaci.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Z., Guo Y., Shi H., et al. Shared genetic and experimental links between obesity-related traits and asthma subtypes in UK Biobank. J Allergy Clin Immunol. Feb 2020;145(2):537–549. doi: 10.1016/j.jaci.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pike K.C., Crozier S.R., Lucas J.S., et al. Patterns of fetal and infant growth are related to atopy and wheezing disorders at age 3 years. Thorax. Dec 2010;65(12):1099–1106. doi: 10.1136/thx.2010.134742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson E.L., Fraser A., Martin R.M., et al. Associations of postnatal growth with asthma and atopy: the PROBIT Study. Pediatr Allergy Immunol. Mar 2013;24(2):122–130. doi: 10.1111/pai.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wandalsen G.F., Chong-Neto H.J., de Souza F.S., Solé D., Bacharier L.B. Early weight gain and the development of asthma and atopy in children. Curr Opin Allergy Clin Immunol. 2014;14(2):126–130. doi: 10.1097/aci.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y.C., Dong G.H., Lin K.C., Lee Y.L. Gender difference of childhood overweight and obesity in predicting the risk of incident asthma: a systematic review and meta-analysis. Obes Rev. Mar 2013;14(3):222–231. doi: 10.1111/j.1467-789X.2012.01055.x. [DOI] [PubMed] [Google Scholar]
- 36.Ekström S., Magnusson J., Kull I., et al. Body mass index development and asthma throughout childhood. Am J Epidemiol. Jul 15 2017;186(2):255–263. doi: 10.1093/aje/kwx081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wandalsen G.F., Borges L.V., Barroso N., et al. Gender differences in the relationship between body mass index (BMI) changes and the prevalence and severity of wheezing and asthma in the first year of life. Allergol Immunopathol. 2015;43(6):562–567. doi: 10.1016/j.aller.2014.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, T-C. Yao, upon reasonable request.