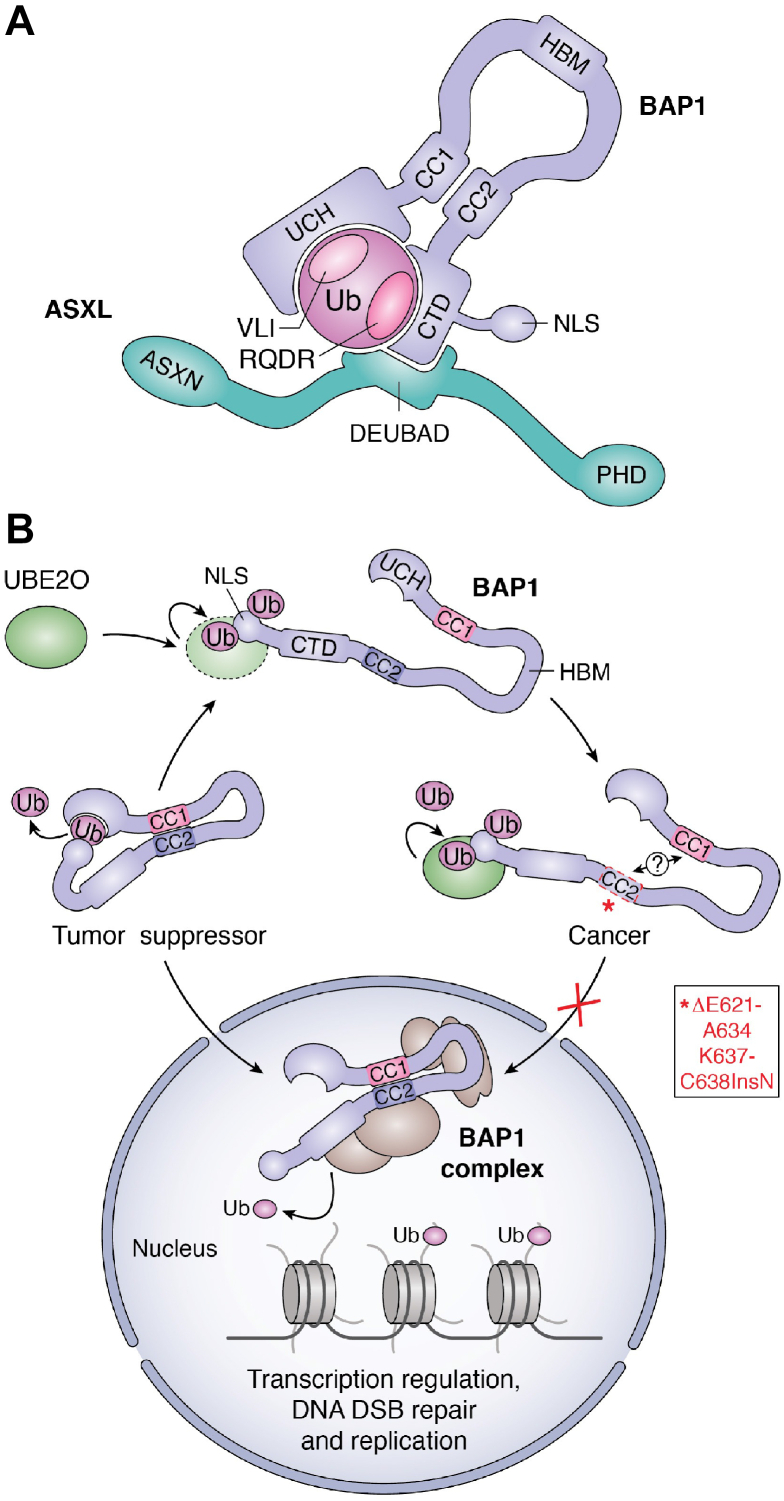
Figure 4.
Regulation of BAP1 by intramolecular interactions and self-deubiquitination. A, BAP1 intramolecular interactions are required for its ASXLs-mediated regulation. Interactions between the UCH-CC1 and the CTD domains of BAP1 as well as between the CTD and the DEUBAD of ASXLs are essential for the formation of the CUBI (composite ubiquitin-binding interface). This composite interface enables binding of the VLI hydrophobic patch and the RQDR charged patch of ubiquitin to BAP1 UCH domain and CTD, respectively. This assembly results in the activation of BAP1 DUB activity. B, regulation of BAP1 by self-deubiquitination. UBE2O is a E2 Ub-conjugating-E3 Ub ligase hybrid enzyme that multimonoubiquitinates BAP1 in its NLS region. The CC2 region of BAP1 interacts with the UCH-CC1 domains. This event brings the NLS close enough to the UCH catalytic domain facilitating NLS deubiquitination. BAP1 is then able to translocate into the nucleus and act as a tumor suppressor. Cancer mutations localized in the CC2 domain promote BAP1 association with UBE2O and lead to the disruption of BAP1 self-deubiquitination. As a consequence, BAP1 is sequestered in the cytoplasm. BAP1, BRCA1-associated protein 1; CC1/2, coiled-coil 1/2; CTD, C-terminal domain; DEUBAD, DEUBiquitinase ADaptator; HBM, HCF-1 binding motif; NLS, nuclear localization signal; PHD, plant homeo-domain; Ub, ubiquitin; UCH, ubiquitin carboxy-terminal hydrolase.