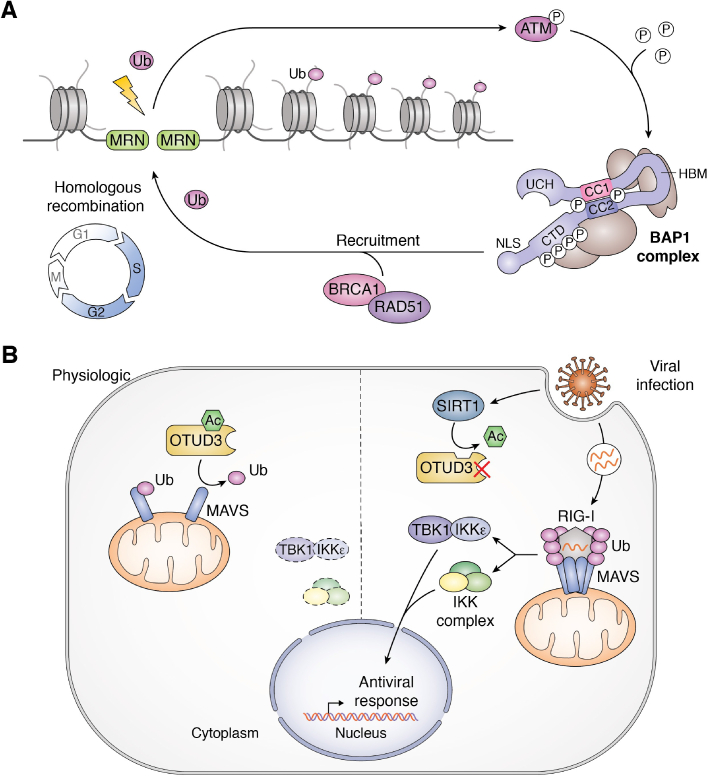
Figure 6.
DUB regulation by post-translational modifications. A, phosphorylation of BAP1 during the DNA damage response. Upon ionizing-irradiation, DNA double-stand breaks (DSBs) are recognized by the MRN complex resulting in ATM activation. ATM then phosphorylates BAP1 leading to its recruitment at the DSB sites. In turn, BAP1 recruits homologous recombination (HR) proteins such as BRCA1 and RAD51 to promote HR. B, acetylation of OTUD3 in innate immunity. Under normal conditions, OTUD3 is acetylated on its lysine 129 (K129-Ac). K129-Ac enhances OTUD3 DUB activity toward the poly-K63 ubiquitin chains of the immune protein MAVS. This mechanism prevents the aggregation of MAVS and activation of the innate immune response. However, upon viral infection, the deacetylase SIRT1 is recruited to OTUD3 to catalyze its deacetylation and inactivation. As a consequence, MAVS becomes polyubiquitinated and interacts with RIG-I, leading to its aggregation. This ultimately leads to the activation of downstream immune signaling pathways involved in the antiviral response. ATM, ataxia telangiectasia mutated; BAP1, BRCA1-associated protein 1; IKK, IκB kinase; MAVS, mitochondrial anti-viral signaling protein; MRN, MRE11/RAD50/NBS1; OTUD3, OTU-domain containing protein 3; RIG-I, retinoic acid-inducible gene I; SIRT1, sirtuin 1; TBK1, TANK-binding kinase 1; Ub, ubiquitin.