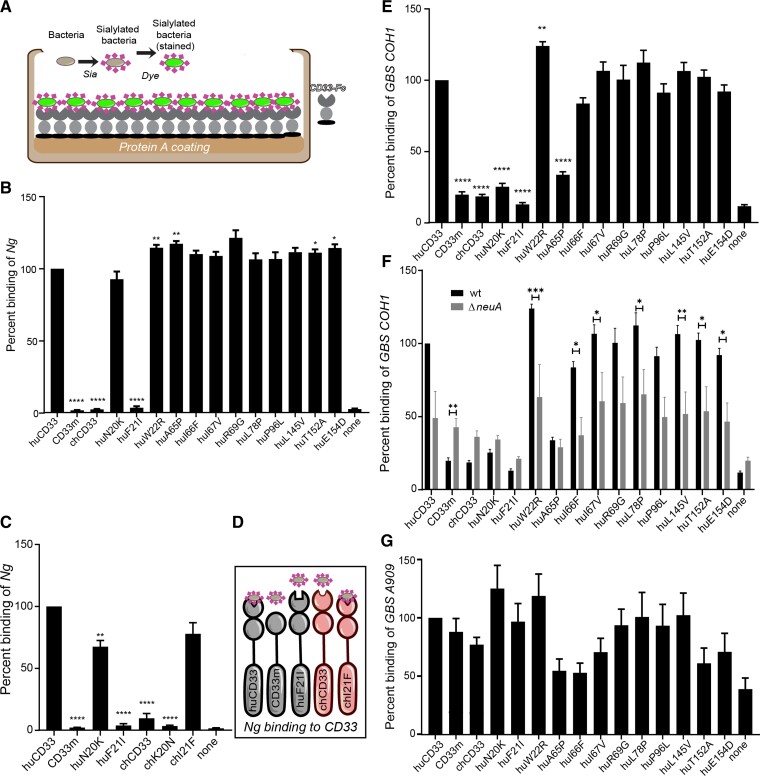
Fig. 2.
Human-specific amino acid changes in CD33 affects bacterial binding. (A) Schematic of the ELISA-based assay using recombinant CD33-Fc chimeric proteins immobilized on protein A coated plates used to determine binding of the sialylated bacteria is shown. (B) Binding of fluorescently labeled Neisseria gonorrhoeae (Ng) was determined. The position of the amino acid different from the wildtype huCD33 protein is indicated below each bar in the x-axis. The bacterial binding to each individual CD33 mutant was normalized to the binding of wildtype huCD33 for that assay. “None” indicates no protein control for the background bacterial binding to the plate. (C) Binding of Ng to immobilized recombinant CD33 proteins containing the corresponding amino acid mutation (position 20 or 21) either in human or chimp CD33 protein backbone. (D) Pictorial summary of Ng binding to CD33 wild type and mutant proteins. (E) Binding of GBS COH1 strain to different CD33 mutant proteins in an ELISA-based assay with immobilized recombinant CD33 proteins. (F) Sialic acid dependence of the binding was determined using wildtype and ΔneuA mutant strains of COH1. (G) Interaction of CD33 proteins among different GBS strains was compared using A909 and COH1 strains. ‘hu’ indicates the corresponding amino acid change in huCD33 backbone and ‘ch’ using chimp CD33. The graphs show the cumulative result from three independent experiments, each done in triplicate. Statistical analysis was performed in Prism software using one-way ANOVA with Durrett post-comparison test. *<0.01, **<0.001, ***<0.0001.