Figure 6. Therapeutic effects of the PARP inhibitor talazoparib in mice bearing ATX-null OvCa.
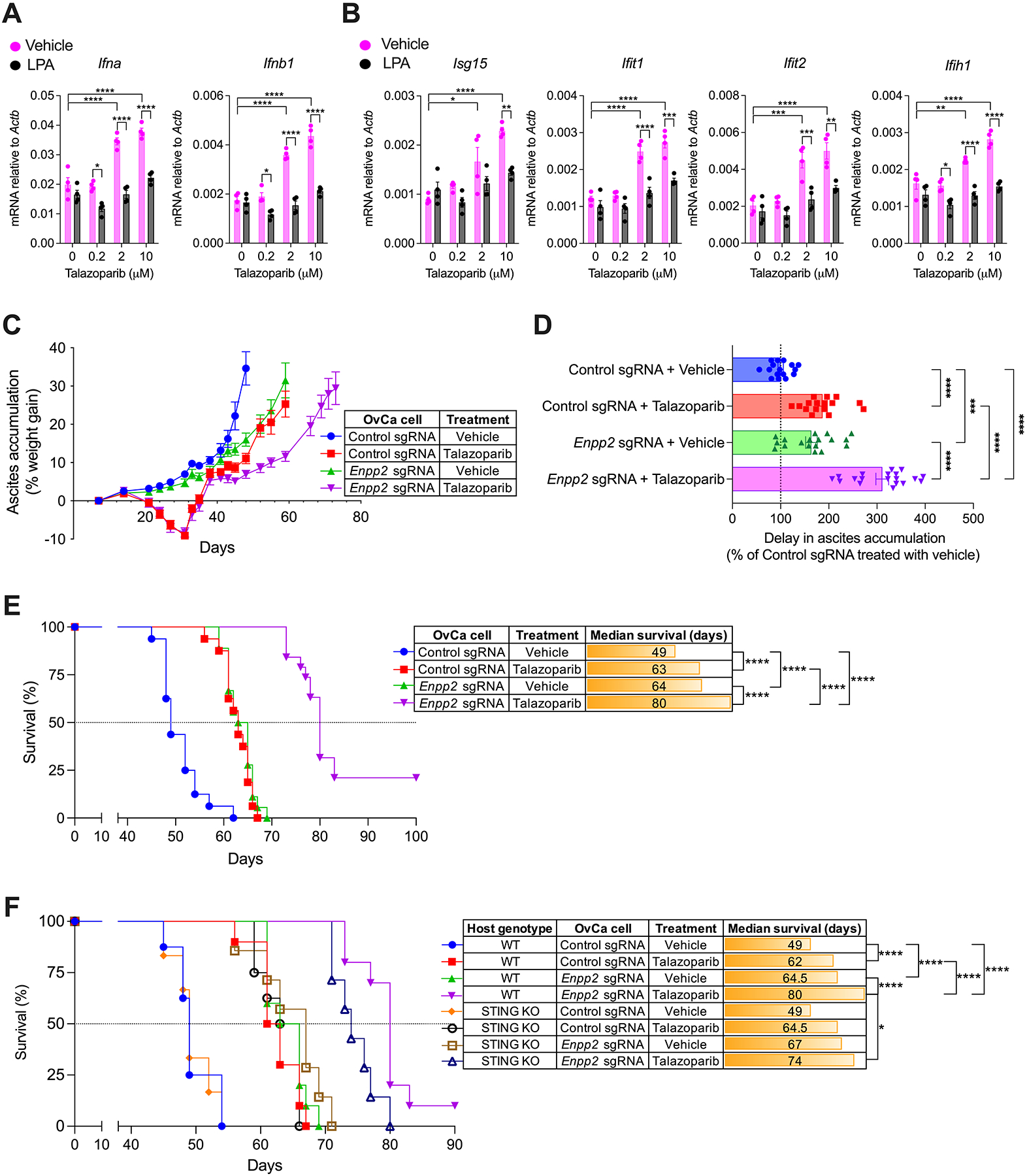
(A and B) RT-qPCR analysis of type-I IFN transcripts (A) and type-I ISGs (B) in BMDCs cocultured with talazoparib-treated OvCa cells in the presence or absence of LPA. Data were normalized to Actb in all cases (n = 4). (C-E) C57BL/6J mice (n = 16–19/group) were challenged via i.p. injection with 1.5 × 106 control or Enpp2-null ID8-Defb29/Vegf-A OvCa cells. After 7 days, mice were treated once daily with vehicle or talazoparib (0.33 mg/kg) by oral gavage for up to 28 days. (C) Ascites accumulation denoted as percent weight gain over time. (D) Changes in ascites development were analyzed by calculating the area above the curve in each experimental group starting on day 35 and a cutoff of 35% of weight gain. Data are represented as percent change compared with control sgRNA group treated with vehicle. (E) Overall survival curves for the same mice described in C and D. (F) Survival experiments were repeated as described in C-E but including STING-deficient (KO) hosts (n = 6–10/group). Data in A-D are shown as mean ± SEM. (A and B), Two-way ANOVA (Tukey’s multiple comparisons test). (D), One-Way ANOVA with Tukey’s multiple comparisons test. (E and F), Log-rank test analysis for survival. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
