Abstract
Human Leukocyte Antigen (HLA) expression contributes to the activation of anti-tumor immunity through interactions with T cell receptors. Pan-cancer HLA-mediated immunogenicity and immunoediting mechanisms have not been systematically studied previously. In a retrospective analysis of 33 tumor types from the Cancer Genome Atlas (TCGA), we characterized the differential expression of HLA class I and class II genes across various oncogenic pathways and immune subtypes. While HLA I genes were upregulated in all immunogenically hot tumors, HLA II genes were upregulated in an inflammatory immune subtype associated with best prognosis and were systematically downregulated in specific oncogenic pathways. A subset of immunogenically hot tumors which upregulated HLA class I but not class II genes exploited HLA-mediated escape strategies. Furthermore, with a machine learning model, we demonstrated that HLA gene expression data can be used to predict the immune subtypes of patients receiving immune checkpoint blockade and stratify patient survival. Interestingly, tumors with the highest immune infiltration did not have the best prognosis but showed significantly higher immune exhaustion.
Implications
Taken together, we highlight the prognostic potential of HLA genes in immunotherapies and suggest that higher tumor immunogenicity mediated by HLA expression may sometimes lead to tumor escape under strong selective pressure.
Introduction
The tumor microenvironment (TME) is a hostile environment for infiltrating immune cells and poses significant challenges to their proper function. Tumors may exploit immunosuppressive strategies to evade immune rejection, including the expression of immune checkpoints like programmed death-ligand 1 (PD-L1) and the recruitment of immune suppressor cells (1). Shaped by the complex interactions between cellular components in the TME, the immune microenvironment varies significantly among patients and across tumor types.
In recent years, significant efforts have been made to study the dynamic components in the TME to shape immunogenically hot tumors, which are associated with better response to therapy (2). In the antigen presentation pathway, the interactions between peptide/major histocompatibility complex (MHC) and T cell receptors (TCR) are critical in triggering adaptive immunity (3). The MHC is highly polymorphic, enabling the presentation of a wide variety of antigens on the cell surface (4). In particular, the classical MHC I molecules (HLA-A, HLA-B, HLA-C) present endogenous antigens to CD8 T cells, and MHC II molecules (HLA-DR, HLA-DQ, HLA-DM) present exogenous antigens to CD4 helper T cells (5,6). The less polymorphic non-classical MHC I molecules (HLA-E, HLA-G) can act as inhibitory ligands to NK cells and contribute to immune tolerance (7). The non-classical MHC II molecules (HLA-DM, HLA-DO) regulate antigen processing and loading as chaperones (8). In the TME, both the generation of tumor-specific antigens (neoantigens) and the expression of antigen-presenting MHC molecules are important for effective immunity. A large pool of neoantigens derived from tumor mutations help trigger T cell response, shaping the formation of hot tumors (9,10). Deep learning algorithms have been built to predict high-affinity MHC-neoantigen binding, which contributes to the development of neoantigen-based cancer vaccines (11). On the other hand, immunohistochemistry (IHC) results have shown that upregulation of Human Leukocyte Antigen (HLA) class I expression in early-stage tumors may lead to CD8 T cell-mediated anti-tumor immunity (12). Inducement of HLA I expression in tumors by pro-inflammatory cytokines or by the inhibition of DNA methyltransferase can also result in strong cytotoxic CD8 T cell response (13,14).
While these studies have demonstrated the potential role of HLA genes and neoantigen presentation in triggering immune-mediated tumor rejection, integrated analysis of HLA genes in shaping various immune microenvironments and tumor evasion has not been performed in a pan-cancer fashion. Here, we aimed to uncover how HLA class I and II are differentially regulated across tumors with alterations of cancer driving genes in specific oncogenic pathways, potentially shaping immune subtypes in the tumor microenvironment. We also assessed the distribution of HLA supertypes and allelic diversity, and their correlations with immunogenicity and prognosis. In addition, we evaluated HLA-mediated tumor escape strategies, including DNA methylation at HLA genes and HLA LOH in different immune subtypes. Our study highlights the potential clinical relevance of HLA genes in predicting immune subtypes and tumor responsiveness to immunotherapies.
Materials and Methods
Study Design
The aim of the study was to systematically examine HLA-mediated tumor immunogenicity and escape across tumor types. HLA class I and II gene expression and correlations with immune infiltration were assessed across TCGA tumor types and their molecular subtypes. The distributions of HLA supertypes and allelic diversity were characterized to investigate how germline HLA heterozygosity correlates with patient survival. Differential expression of HLA genes across oncogenic pathways and immune subtypes was identified. In addition, we considered DNA methylation at HLA genes and HLA LOH as potential HLA-mediated tumor escape strategies to shape immunogenicity. To test the generalizability of HLA gene expression as a predictor of immune subtypes, a machine learning model was trained on TCGA SKCM HLA gene expression data and applied to predict the immune subtypes in an immunotherapy-treated melanoma cohort.
Study Cohorts and Data Acquisition
The samples analyzed in this study include primary tumors of 33 TCGA tumor types (ACC: adrenocortical carcinoma, BLCA: bladder urothelial carcinoma, BRCA: breast invasive carcinoma, CESC: cervical squamous cell carcinoma and endocervical adenocarcinoma, CHOL: cholangiocarcinoma, COAD: colon adenocarcinoma, DLBC: lymphoid neoplasm diffuse large b-cell lymphoma, ESCA: esophageal carcinoma, GBM: glioblastoma multiforme, HNSC: head and neck squamous cell carcinoma, KICH: kidney chromophobe, KIRC: kidney renal clear cell carcinoma, KIRP: kidney renal papillary cell carcinoma, LAML: acute myeloid leukemia, LGG: brain lower grade glioma, LIHC: liver hepatocellular carcinoma, LUAD: lung adenocarcinoma, LUSC: lung squamous cell carcinoma, MESO: mesothelioma, OV: ovarian serous cystadenocarcinoma, PAAD: pancreatic adenocarcinoma, PCPG: pheochromocytoma and paraganglioma, PRAD: prostate adenocarcinoma, READ: rectum adenocarcinoma, SARC: sarcoma, SKCM: skin cutaneous melanoma, STAD: stomach adenocarcinoma, TGCT: testicular germ cell tumors, THCA: thyroid carcinoma, THYM: thymoma, UCEC: uterine corpus endometrial carcinoma, UCS: uterine carcinosarcoma, UVM: uveal melanoma).
HT-Seq FPKM reads of RNA-seq expression data, methylation array files from Illumina Infinium Human Methylation 450k (HM450), and whole-exome sequencing bam files of tumors and matched tumor-adjacent normal samples were obtained from GDC Data Portal (https://portal.gdc.cancer.gov/). Briefly, for the gene-level RNA-Seq expression data, the GDC workflow includes aligning the reads to GRCh38 using a two-pass method with STAR (https://docs.gdc.cancer.gov/Data/Bioinformatics_Pipelines/Expression_mRNA_Pipeline/). HT-Seq was used to calculate the raw counts of genes annotated by GENCODE v22. FPKM was calculated from the expression raw counts.
For HLA expression analysis, DLBC and LAML were excluded since tumor purity could not be inferred based on immune and stromal fractions. For analysis of HLA expression across molecular subtypes, 9120 patients with defined tumor molecular subtypes were included. For analysis of HLA differential expression between tumor and matched normal tissues, 24 tumor types with available RNA-seq expression data of normal tissues were included. For analysis of HLA allelic divergence, LAML, ESCA, OV were excluded due to insufficient coverage at HLA class I loci for typing.
For the immune checkpoint blockade-treated melanoma cohort (Pembrolizumab: n=71, Nivolumab: n=51), clinical information was obtained from Liu et al. (15). The RNA-sequencing files were downloaded from dbGaP (accession phs000452.v3.p1). FeatureCounts (16) was used to compute the raw counts of mapped genes.
HLA Gene Expression
To represent gene expression, tissue-normalized FPKM value at each gene X was calculated as
Where ∑x FPKM represents the sum of FPKM reads across all genes in a given sample, and represents the tissue-specific sample average of the sum of FPKM reads across all genes. The sample average was measured in each TCGA tumor type and the matched normal tissue, and was used to normalize the gene expression data of the sample belonging to that tissue type.
Since HLA I genes are ubiquitously expressed, high impurities due to immune infiltration and non-tumor cells in the microenvironment may significantly pollute the expression data. We used ESTIMATE (17) to compute an ESTIMATE score for each tumor sample and matched normal tissue, respectively, based on immune fraction and stromal fraction. Purity was calculated using the formula proposed by Yoshihara et al.: purity = cos(0.6049872018 + 0.0001467884 × ESTIMATE score) (17). Normalized expression of HLA I genes was represented as Gene x × purity.
Individual gene expression was represented as the log-transformed expression value. HLA class I gene expression was represented as the log-transformed geometric mean of B2M, HLA-A, HLA-B, and HLA-C expression. HLA class II gene expression was represented as the log-transformed geometric mean of HLA-DRA, HLA-DRB1, HLA-DQA1, HLA-DQB1, HLA-DPA1, and HLA-DPB1 expression.
HLA Typing
The patient-specific 4-digit HLA types were acquired from Thorssen et al. (1), who used OptiType software (18) taking tumor RNA-seq fastq files as input. The HLA types of patients not typed previously were inferred using HLA-HD (19) taking whole-exome sequencing BAM files as input.
HLA Gene Methylation in Tumor and Normal Samples
The HLA class I genes' promoter region was defined as 400 bp upstream from the transcription start site (TSS) to 500 bp downstream of the TSS, covering the first exon based on the gene annotation (20). The gene body region was defined as 500 bp downstream of the TSS to the end of the gene. To identify differentially methylated HLA genes, the methylation difference of HLA genes between the tumor and the paired normal sample was calculated, where HLA methylation level was represented as the mean β-scores across all CpGs in the promoter region and the gene body, respectively. An HLA gene was hypermethylated if the methylation difference of the gene was in the top 25% of all samples, and was hypomethylated if the methylation difference was in the bottom 25% of all samples.
Molecular Subtypes, MSI Status, and Alterations in Oncogenic Pathways
The 60 molecular subtypes for 31 tumor types, as well as their tissue of origin, were obtained from Sanchez-Vega et al. (21). The Microsatellite instability (MSI) status of COAD (n=262), STAD (n=292), and UCEC patients (n=257) were obtained from Cortes-Ciriano (22). Alterations in 10 oncogenic pathways (cell cycle, Hippo, Myc, Notch, NRF2, PI3K, RTK/RAS, TGF-β, TP53, WNT) for 9125 patients were obtained from Sanchez-Vega et al. (21). In brief, possible driver cancer genes involved in these pathways were curated by the authors based on previous published works. Recurrent alterations, including mutations, gene fusions, copy-number alterations and epigenetic alterations, were examined. A tumor with at least one alteration in the driver genes of a specific pathway was classified as altered in that pathway (21).
Immune Subtypes, Immune Cellular Fraction Estimates, Tumor Mutational Burden, and Cytolytic Activity.
The immune subtypes, immune cellular fraction estimates, tumor mutational burden, and neoantigen load were obtained from Thorssen et al. (1). In brief, five immune signature modules were identified from 160 immune expression signatures, including macrophages/monocytes (23), overall lymphocyte infiltration (24), TGF-β response (25), IFN-γ response (26), and wound healing (27). These modules were further used to cluster 6 resulting immune subtypes C1-C6. Immune cellular fraction estimates of 22 immune cell types were inferred with CIBERSORT (28) using TCGA RNA-Seq data. Th1 score was calculated based on the Th1 gene signature (1). For tumor mutational burden, protein-coding somatic mutation including insertions/deletions, missense mutations, nonsense mutations, frameshift mutations, nonstop mutations, splice site mutations, and transcription start site mutations were called (1). Non-silent mutation rate per Mb was calculated. For neoantigen prediction, neoepitopes were inferred from single nucleotide variants (SNVs) and insertion-deletion mutations (Indels) using NetMHCpan v3.0 (29). Mutant peptides were identified as potential neoantigens if the predicted binding affinity (IC50) to autologous MHC < 500 nM and gene expression > 1.6 transcripts-per-million (TPM) (1).
Cytolytic activity in each tumor sample was represented as cytolytic score (CYT) as previously proposed (30). The expression levels of GZMA and PRF1 were used. The following formula was applied to represent CYT:
Functional Divergence of HLA Alleles and Patient HED
HED, which represents the germline HLA allelic diversity, was calculated as previously described (31). In brief, protein sequences of HLA alleles specific to each patient and exon annotations were obtained from the ImMunoGeneTics/HLA (IMGT/HLA) database (https://www.ebi.ac.uk/ipd/imgt/hla/). Protein sequence of exons 2 and 3, namely the peptide-binding domain, of each HLA allele was extracted. Pairwise alignment between the protein sequences of two alleles was performed using MUltiple Sequence Comparison by Log-Expectation (MUSCLE) (32). The Grantham distance, which represents the functional divergence between two alleles, was calculated as the sum of amino acid differences (including the biochemical composition, polarity, and volume of each amino acid) in the pairwise alignment following the formula by R. Grantham (33):
where D is the Grantham distance between the aligned sequences, and i and j are the amino acids at a homologous position. c, p, and v represent biochemical composition, polarity, and volume of the amino acids, respectively. α, β, and γ are constants as originally proposed. The Grantham distance was normalized by the length of the sequence alignment. Patient HED was calculated as the mean of Grantham distances at HLA-A, HLA-B, and HLA-C.
Classification
To predict the immune subtypes of all tumors, one-hot encoding was applied to TCGA tumor types; the log 2 values of classical and non-classical HLA class I (HLA-A, HLA-B, HLA-C, HLA-E, HLA-G), B2M, and class II gene (HLA-DRA, HLA-DRB1, HLA-DQA1, HLA-DQB1, HLA-DPA1, HLA-DPB1, HLA-DMA, HLA-DMB, HLA-DOA, HLA-DOB) expression in tumors were used to train a random forest classifier with n_estimators=200 and criterion=‘gini’.
For cross-validation, the dataset was divided into training sets (60%) and testing sets (40%) randomly. Area under the curve (AUC) was calculated. The process was repeated 10,000 times.
The Liu anti-PD1-treated melanoma cohort (15) was used for validation. Normalized gene expression was calculated as for the TCGA and validation dataset, respectively, where normalization score was the log-average of a housekeeping gene signature (C1orf43, CHMP2A, EMC7, GPI, PSMB2, PSMB4, RAB7A, REEP5, SNRPD3, VCP, VPS29) (34). The random forest classifier was trained on all TCGA SKCM patients (C1: n=41, C2: n=27, C3: n=14, C4: n=19, C6: n=2) to predict the immune subtypes of patients in the validation dataset. Cross-validation of the classifier was performed the same way as described above.
HLA Loss of Heterozygosity (LOH) Analysis
LOHHLA (Loss of Heterozygosity in Human Leukocyte Antigen) (35) was used to assess HLA LOH in BRCA, LUAD, and SKCM patients. The program requires a tumor and germline BAM, patient-specific HLA typing, HLA allele sequence FASTA file, and purity and ploidy estimates. Purity and ploidy estimates were acquired from GDC (https://portal.gdc.cancer.gov/). A patient was identified as fully heterozygous if the individual has distinct alleles at all loci of HLA-A, HLA-B, HLA-C. HLA LOH was defined as an allele’s copy number < 0.5 with p value ≤ 0.05.
Statistics and Survival Analysis
Statistical analyses were performed in R v.4.0.4 (RRID:SCR_001905). Wilcoxon Rank Sum Test and Kruskal-Wallis Test were used for comparison of a variable of interest in two groups and multiple groups, respectively. Two-sided Fisher’s Exact Test was used to determine the significance of any difference in proportions between two classifications. Spearman’s rank correlation was used to determine the correlation of two variables.
The values of progression-free interval (PFI) and status were used as obtained from Liu et al. (36). To examine the survival effect of a continuous variable x, samples were split equally into high-x and low-x patients and analyzed using log-rank test and Kaplan-Meier estimator. Cox proportional hazards modeling was used to assess the correlation between multiple variables and patient survival.
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Rachel Karchin (karchin@jhu.edu).
Material Availability
This study did not generate new unique reagents.
Data and Code Availability
This paper analyzes existing, publicly available data. Gene expression data and downstream analysis data are available within the article and its supplementary data files.
All original code has been deposited at https://github.com/KarchinLab/HLA-Mediated-Tumor-Immunogenicity-Manuscript and is publicly available as of the date of publication.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Results
Overview of HLA Gene Expression across TCGA Tumors.
We first assessed individual HLA class I and class II gene expression, as well as Beta-2-microglobulin (B2M) in association with MHC I heavy chains, among the molecular subtypes of 31 TCGA tumor types originated from 13 tissue sites. Since most HLA I genes are ubiquitously expressed, the expression calculated from bulk RNA-seq was corrected by a purity score to account for the heterogeneity of cell types in tumors and matched normal tissues (Methods). Overall, HLA expression showed more variability between genes than between tissue sites. B2M and most HLA class I genes demonstrated higher expression than class II genes (Figure 1A). Consistent with the fact that HLA-G and HLA-DO molecules have more tissue-restricted distribution (37,38), we observed the lowest expression in HLA-G and HLA-DO (HLA-DOA, HLA-DOB) among class I and class II genes, respectively.
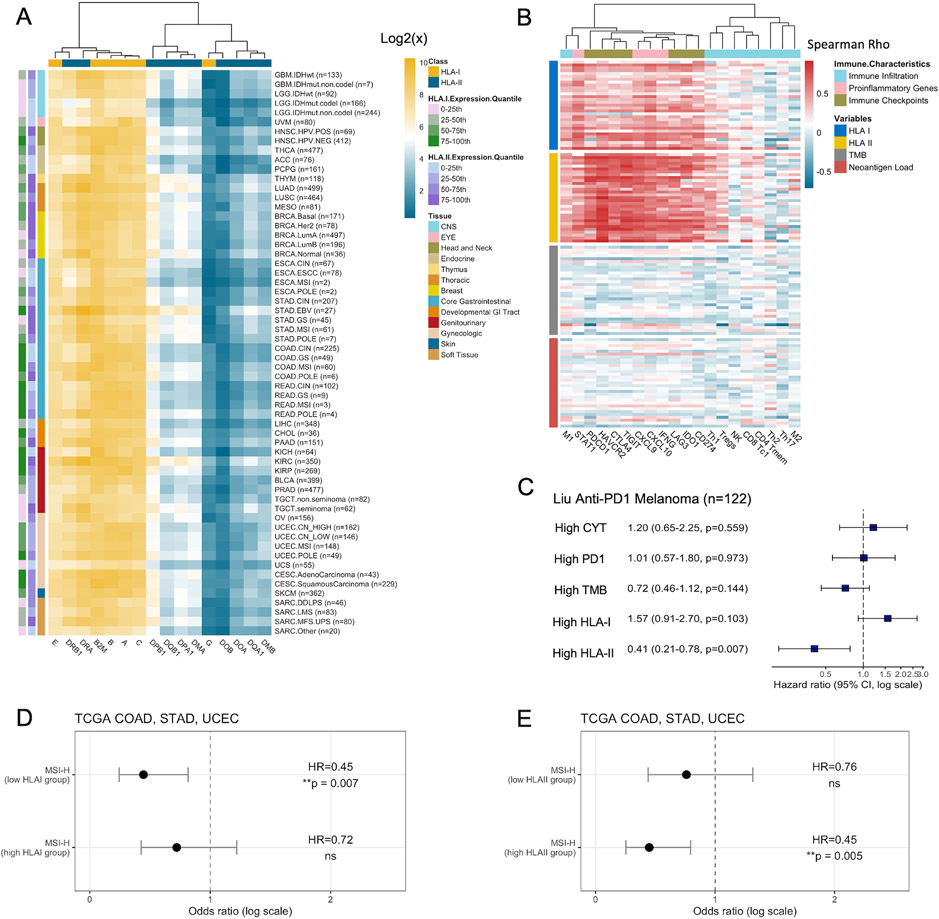
Figure 1. HLA Class I and Class II Expression across Tumor Types.
(A) Expression of classical and non-classical HLA I and II genes across 60 molecular subtypes in 31 TCGA tumor types. Expression of gene X is represented as the mean gene expression of all samples within the tumor type. (B) Spearman correlations between HLA class I, class II expression, tumor mutation burden (TMB), neoantigen load, and immune characteristics. Each row within a horizontal block represents a distinct tumor type. CD8 Tc1: CD8 cytotoxic T cells; CD4 Tmem: activated CD4 memory T cells; Th1: T helper 1 cells; Th2: T helper 2 cells; Th17: T helper 17 cells; Tregs: regulatory T cells; NK: natural killer cells; M1: Macrophage 1; M2: Macrophage 2. (C) The hazard ratio (HR) of CYT, PDCD1 expression, TMB, HLA I expression, and HLA II expression (split by the median) on PFS from Cox Proportional-hazards model in the Liu anti-PD1 melanoma cohort (n=122). Squares denote the HR and horizontal bars represent 95% confidence intervals. (D) The hazard ratio (HR) of the effect of MSI-H on patient PFS among high HLA-I expressing tumors (top 50%) and low HLA-I expressing tumors from log-rank test. (E) Same as (D), but for HLA-II expression.
We then evaluated whether HLA expression was associated with several immune-related tumor characteristics, including immune infiltration, proinflammatory gene signatures, and immune checkpoint signatures. Compared to tumor mutational burden (TMB) and neoantigen loads (11,39), HLA I and II expression demonstrated a stronger correlation with infiltration of anti-tumor immune cells, including macrophage 1 (M1), CD8 cytotoxic T cells (CD8 Tc1), activated CD4 memory T cells (CD4 Tmem), natural killer cells (NK), regulatory T cells (Treg) and T helper 1 cells (Th1), but not the pro-tumor macrophage 2 (M2) or the immunosuppressive T helper 2 cells (Th2) (Figure 1B). They were also positively correlated with the expression of pro-inflammatory genes, as well as immune checkpoints. Among the classical and non-classical HLA I genes, HLA-C demonstrated the highest correlation with cytolytic activity (CYT) (Spearman correlation, R=0.52) (30), which measures Granzyme A (GZMA) and Perforin-1 (PRF1) expression to represent CD8 T cell cytotoxicity (Figure S1A). HLA-G showed the lowest correlation with CYT (R=0.32). Among HLA II genes, HLA-DQ and HLA-DR showed the highest correlation with CYT (R=0.73), while HLA-DM had the lowest (R=0.64). An unsupervised principal-component analysis (PCA) of all HLA I and HLA II expression data showed that tumors with high CYT clustered together (Figure S1B).
Interestingly, while both HLA I and II expression correlated positively with immunogenicity, only patients with high HLA II (top 50%) showed better survival (HLA I: HR=0.94, p=0.053, HLA II: HR=0.90, p=0.002). In addition, among a cohort of melanoma patients receiving anti-PD1 immune checkpoint blockade (ICB) acquired from Liu et al. (15), the pre-treatment HLA II expression was also more predictive of patient survival (Cox proportional hazards, HR=0.41, p=0.007) than HLA I, as well as TMB, immune checkpoint (PDCD1) expression, and CYT (Figure 1C).
Microsatellite instability-high (MSI-H) tumors have higher mutational loads due to defects in mismatch repair (MMR) and better prognosis in general (40). We evaluated HLA expression across COAD, STAD, and UCEC with various MSI status. While HLA I showed no difference in expression between MSI-H, MSI-L and MSS tumors, HLA II showed significantly higher expression in MSI-H COAD (Kruskal-Wallis, p=4.5e-5) and STAD tumors (p=0.012) (Figure S1C-D). Interestingly, MSI-H patients with low HLA I expression correlated with significantly better progression free survival (PFS) (Log-rank test, HR=0.45, p=0.007) than those with high HLA I expression (HR=0.72, p=0.22) (Figure 1D). In contrast, MSI-H patients with high HLA II expression showed better PFS (HR=0.45, p=0.005) than low HLA II expression (HR=0.76, p=0.33) (Figure 1E). Our results suggest that high expression of HLA I and II molecules might facilitate the infiltration of different types of immune cells, leading to contrasting impacts on survival in MSI-H tumors.
HLA Allelic Diversity in Tumors with High HLA Expression Correlates with Improved Survival.
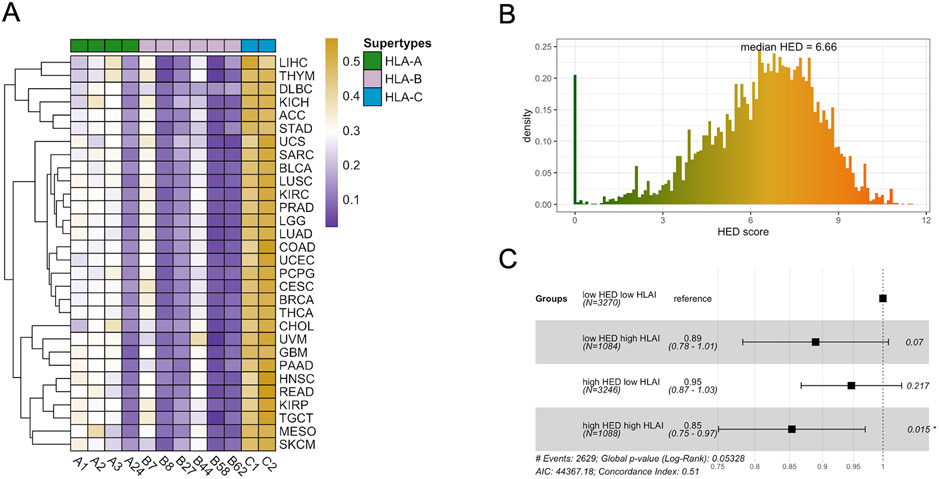
MHC-I molecules are highly variable with divergent biophysiochemical properties in the peptide-binding grooves to present antigens. We next assessed the distribution of HLA class I supertypes (41,42) among TCGA tumors. Overall, the supertypes showed an unbiased distribution across tumor types (Figure 2A). A24 was the least predominant HLA-A supertypes, while B8, B27, B58 and B62 were least predominant among HLA-B supertypes.
Figure 2. HLA Supertypes and Heterozygosity.
(A) Frequencies of HLA class I supertypes among tumor types. Color scales represents the frequency of occurrence for each supertype in the corresponding tumor types. (B) HED score distribution from 30 tumor types (n=8859). (C) The hazard ratio (HR) of the effects of HED (high: top 50%) and HLA I expression (high: top 25%) on PFS from Cox Proportional-hazards model, with low HED low HLA-I expression as reference. Squares represent the HR and horizontal bars represent 95% confidence intervals.
Since a higher diversity of a patient's HLA alleles could potentially allow a larger pool of neoantigens to be presented and hence increase the likelihood of the tumor being rejected by immunity (31), we asked if the germline HLA allelic diversity is correlated with tumor immunogenicity. The allelic diversity of each patient's HLA class I alleles was estimated with the HLA-I evolutionary divergence (HED) score (43). A patient homozygous at all three HLA I loci has a HED score of 0. The greater the functional diversity of HLA I alleles, the higher the patient's HED score (Figure S2A). The median HED of all patients was 6.66, with a low being 0 and a high being 11.54 (Figure 2B). However, we did not observe any correlations between HLA I allelic diversity and infiltration levels of immune cells, pro-inflammatory genes, or immune checkpoints in the TME (Figure S2B). Interestingly, high HED scores in patients with the highest HLA I expression demonstrated significant correlation with better survival than HLA I or HED alone (Cox proportional hazards, 0.85, p=0.015) (Figure 2C). We reasoned that high allelic divergence of HLA I alleles in tumors expressing high levels of HLA genes may more efficiently present neoantigens and lead to tumor rejection.
HLA Differential Expression across Immune Subtypes and Oncogenic Pathways.
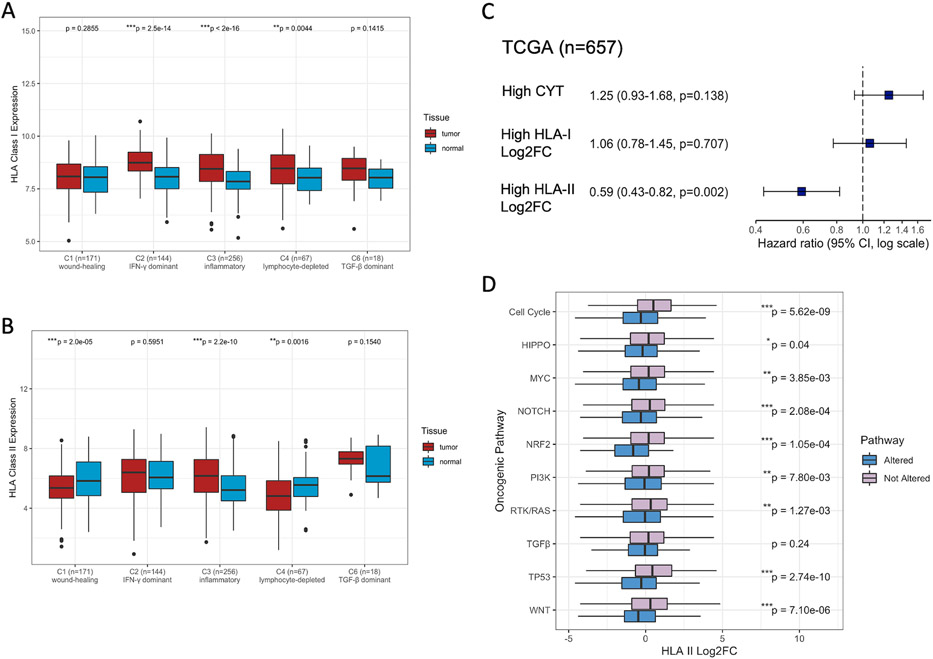
Next, we examined the differential expression of HLA genes between the tumors and paired normal tissues across different immune microenvironments. To this end, we utilized six immune subtypes (C1-C6) previously clustered from 160 immune expression signatures, to our knowledge excluding HLA genes: C1 (wound healing), C2 (IFN-γ dominant), C3 (inflammatory), C4 (lymphocyte depleted), C5 (immunologically quiet), and C6 (TGF-β dominant) (1). C5 is characterized with the lowest level of immune infiltrates, while C4 and C6 have the worst prognosis with a macrophage-dominated TME (1). Notably, while C2 has the highest levels of immune infiltration and intratumoral heterogeneity (ITH), C3 correlates with the best prognosis (1). Overall, we found that HLA I expression was the most significantly upregulated in C2 (Wilcoxon signed-rank, p=2.5e-14) and C3 (p<2e-16) (Figure 3A). HLA II expression was significantly downregulated in C1 (p=2.0e-5) and C4 (p=0.0016) (Figure 3B). Surprisingly, though both C2 and C3 were immunogenically hot, HLA II was uniquely upregulated in C3 (p=2.2e-10). Differential expression of HLA genes of each immune subtype was variable across cancer types, potentially due to the limitation of small sample sizes and the heterogeneity of HLA differential regulation across cancers (Figure S3A-B, C-D). For instance, KIRC showed the most HLA upregulation, while COAD and LUSC showed the most HLA I and II downregulation, respectively. As HLA expression machinery defects are associated with cancer progression in many tumor types, KIRC has been shown to have low frequency of such defects with high success rate of immunotherapy (44). Hence, functional antigen presenting machinery plays a crucial role in triggering anti-tumor immunity.
Figure 3. HLA Differential Expression Associated with Immune Subtypes and Oncogenic Pathways.
(A) HLA class I expression, and (B) HLA class II expression between tumors and matched normal samples across five immune subtypes (excluding C5 with no available data). P-values between pairwise tumor-normal samples are calculated by Wilcoxon signed-rank test. Asterisks denote significant FDR-adjusted (Benjamini & Hochberg method) p-values (*p<0.05; **p<0.01; ***p<0.001). (C) The hazard ratio (HR) of CYT, HLA-I Log2FC, and HLA-II Log2FC (split by the median) on PFS from the Cox Proportional-hazards model. Squares denote the HR and horizontal bars represent 95% confidence intervals. (D) HLA-II log2 fold change (Log2FC between tumors and matched normal samples) across oncogenic pathways. P-values between tumors with alterations in each oncogenic pathway and those without are calculated by Wilcoxon rank-sum test and are unadjusted. Asterisks denote significant FDR-adjusted (Benjamini & Hochberg method) p-values.
Importantly, patients with high HLA-II fold change demonstrated significantly elevated Th1 signature scores than those with low HLA-II fold change (Figure S3E). On the other hand, high HLA-I and HLA-II fold change both correlated with increased CD8 T cell infiltration (Figure S3F). High HLA II fold change predicted significantly better patient prognosis (Cox proportional hazards, HR=0.59, p=0.002) than high HLA I fold change (HR=1.06, p=0.71) or CYT (HR=1.25, p=0.138) (Figure 3C). While the upregulation of HLA I and II genes was associated with cytotoxic T cell-mediated immunity, our results suggest a strong correlation between HLA II upregulation and helper T cell-mediated immunity, which might lead to more effective T cell priming in the TME.
Alterations in oncogenic pathways may trigger the dysregulation of genetic programs and shape the immune repertoire (1). We next asked how HLA genes were differentially expressed in tumors with alterations in each of the 10 oncogenic signaling pathways (cell cycle, Hippo, Myc, Notch, NRF2, PI3K, RTK/RAS, TGF-β, TP53, WNT) previously compiled by Sanchez-Vega et al. (21). While HLA I differential expression showed no difference except in the WNT pathway, HLA II genes were systematically downregulated in multiple pathways, including the cell cycle, Hippo, Myc, Notch, NRF2, PI3K, RTK/RAS, TP53, and WNT pathway (Figure 3D, Figure S3G). Taken together, these results suggest that alterations in oncogenic pathways may lead to insufficient T cell priming mediated by HLA II downregulation, resulting in worse prognosis.
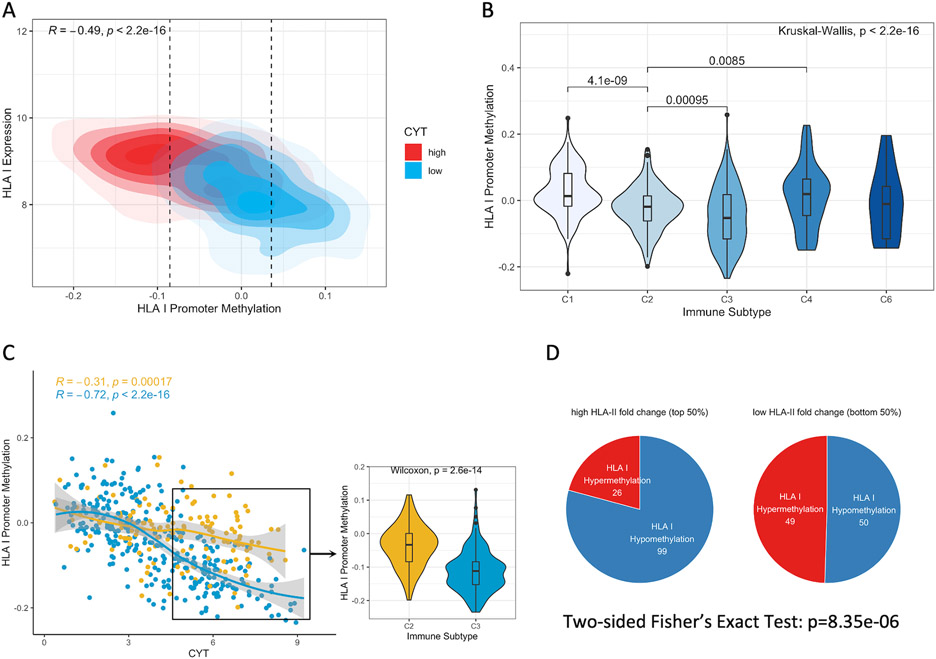
DNA Methylation near HLA Class I Genes Favored under Strong Cytolytic Activity Can Be Alleviated by Class II Gene Upregulation.
Next, we considered DNA methylation of HLA genes as a potential HLA-mediated tumor escape strategy. Overall, there was a significant inverse correlation between HLA I expression and differential methylation at HLA promoter regions (Spearman correlation, R=−0.49, p<2.2e-16) and gene bodies (R=−0.38, p<2.2e-16) (Figure 4A, S4A) (13). Tumors with high cytolytic activity were more hypomethylated, whereas those with low cytolytic activity were hypermethylated. Consistent with low HLA I gene expression observed in C1 (wound healing) and C4 (lymphocyte depleted), HLA I promoter methylation was elevated in C1 and C4 (Kruskal-Wallis, p<2.2e-16) (Figure 4B). Surprisingly, C2 (IFN-γ dominant) demonstrated significantly higher HLA I promoter methylation compared to C3 (inflammatory) (Wilcoxon rank-sum, p=9.5e-4), though their HLA I expression fold change was comparable (Figure S4B). This suggests that while tumors in both C2 and C3 upregulated HLA I expression, C2 tumors were at the same time heavily methylated near HLA I genes.
Figure 4. HLA Class I Methylation Is Favored in A Subset of Hot Tumors and Dampens Immune Activity.
(A) A kernel density plot of HLA I promoter methylation (tumor – normal) and expression across groups of high (top 50%) or low cytolytic activity (CYT). Correlation coefficient and p-values are calculated from Spearman's rank correlation. Hypomethylation: bottom 25%; Hypermethylation: top 25%. (B) HLA promoter methylation across five immune subtypes (excluding C5 with no available data). P-values between pairwise immune subtypes are calculated by Wilcoxon rank-sum test. P-value across all immune subtypes is calculated by the Kruskal-Wallis test. (C) Left panel: The Spearman correlations between CYT and HLA I promoter methylation in C2 and C3 tumors. Right panel: HLA I promoter methylation in tumors with high CYT across C2 and C3. P-value is calculated by Wilcoxon rank-sum test. (D) Association between HLA II fold change and aberrant methylation states at HLA I promoters in all samples. Numbers on pie charts indicate the number of tumor samples showing hypermethylated or hypomethylated HLA I genes. P-value is calculated from two-sided Fisher's Exact Test.
We focused on tumors in C2 and C3, which were both defined by Type I immune response (1). High-CYT C2 tumors showed heavier HLA I promoter methylation than C3 tumors (Wilcoxon rank-sum, p=2.6e-14) (Figure 4C). Interestingly, HLA II upregulation demonstrated correlation with HLA I promoter hypomethylation (Two-sided Fisher’s Exact Test: p=8.35e-6) (Figure 4D). Our results suggest that under strong immune activity, C2 tumors with significant HLA I upregulation but not HLA II upregulation might increase HLA I promoter methylation at the same time to evade recognition. Upregulation of HLA II genes might be a mechanism to reduce tumor escape and trigger effective immunity in the TME.
HLA Loss of Heterozygosity (LOH) Is Associated with Worse Survival but Can Be Counterbalanced by High HLA Expression.
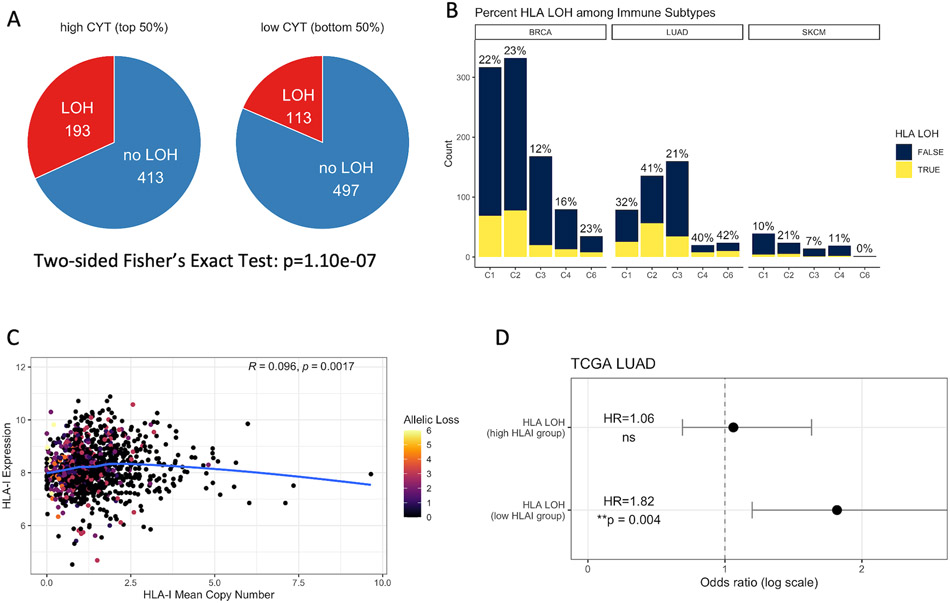
We then considered HLA LOH, another potential HLA-mediated tumor escape strategy, and evaluated how it might correlate with HLA expression and patient survival. We focused on BRCA, LUAD and SKCM, three common cancer types with potential for immunotherapy. Tumors with high cytolytic activity harbored more HLA LOH than those with low cytolytic activity (Two-sided Fisher’s exact Test, p=1.10e-7) (Figure 5A). Across the immune subtypes, tumors in C2 (IFN-γ dominant) demonstrated the highest percentage of HLA LOH, while tumors in C3 (inflammatory) had the lowest (BRCA C2: 23%, C3: 12%; LUAD C2: 41%, C3: 21%; SKCM C2: 21%, C3: 7%) (Figure 5B). Consistent with our observations of HLA I methylation, though the tumors in both C2 and C3 were characterized with Type I immune response, more C2 tumors harbored HLA LOH, suggesting potential immunoediting and tumor escape. HLA LOH correlated with worse patient survival in LUAD (Log-rank test, HR=1.42, p=0.020) but not in BRCA and SKCM (BRCA: p=0.10; SKCM: p=0.20) (Figure S5A-C).
Figure 5. HLA LOH Is Exploited by Hot Tumors with Worse Patient Survival.
(A) Association between cytolytic activity and HLA LOH. Numbers on pie charts indicate the number of tumors harboring HLA LOH. Patients with heterozygosity at all three HLA-I loci are included. P-value is calculated from two-sided Fisher's Exact Test. (B) Tumor samples harboring HLA LOH across five immune subtypes (excluding C5 with no available data) in BRCA (n=932), LUAD (n=419) and SKCM (n=98), respectively. Percentage on top of each bar represents the percent of tumors with HLA LOH within the corresponding immune subtype. (C) Spearman correlation between the mean copy number of HLA class I genes and HLA class I expression. The allelic loss represents the number of lost HLA I alleles. (D) The hazard ratio (HR) of the effect of HLA LOH on LUAD patient PFS among high HLA-I expressing tumors (top 50%) and low HLA-I expressing tumors from the log-rank test. Circles represent the HR and horizontal bars represent 95% confidence intervals.
Surprisingly, we did not find any correlation between the mean HLA I copy number and gene expression (Spearman correlation, R=0.096, p=0.0017) (Figure 5C, S5D-F). We also did not find association between HLA I expression or expression fold change and LOH (Figure S5G-H). This suggests that HLA I expression at the RNA level was uncorrelated with HLA I allelic loss at the DNA level. HLA LOH most likely did not downregulate total HLA expression as a means of facilitating tumor escape.
Based on the notion that HLA LOH may lead to a loss of the most immunogenic neoantigens being presented on the cell surface and result in worse survival (35), we explored if higher HLA gene expression may compensate for reduced neoantigen presentation. Interestingly, we observed worse patient survival in low HLA-I expressing tumors with HLA LOH (Log-rank test, HR=1.82, p=0.004) but not in high HLA-I expressing tumors (HR=1.06, ns) (Figure 5D). We concluded that the correlation between HLA LOH and worse survival may be counterbalanced by higher HLA expression.
Machine Learning Model Predicts the Immune Subtypes with Prognostic Potential.
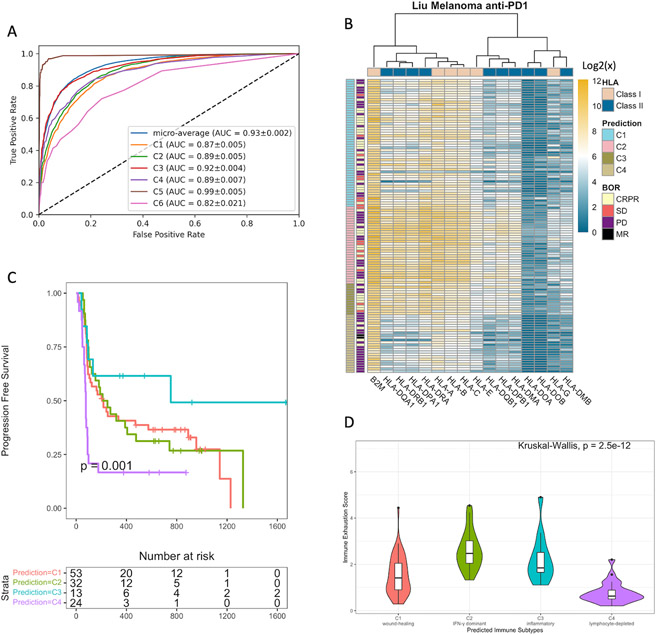
Lastly, we asked if an HLA gene signature could be used to predict the immune subtype of a given tumor sample. A pan-cancer random forest classifier was trained on the transcriptomic data of HLA class I and class II genes to predict the six immune subtypes (Methods). The model predicted the immune subtype of tumors with high accuracy (micro-average AUC=0.93) (Figure 6A). Among the six immune subtypes, tumors in C3 and C5 were classified with the highest accuracy (C3: AUC=0.92, C5: AUC=0.99), while tumors in C6 were classified with the lowest accuracy (C6: AUC=0.82). We then evaluated the generalizability of using the HLA gene signature to predict the immune subtypes. A random forest classifier was trained on HLA expression data from 103 TCGA patients and applied to the pre-treatment HLA expression of patients from the Liu anti-PD1 melanoma cohort (15) (Methods, Table S1). The predicted C2 and C3 showed the highest HLA gene expression, while C4 showed the lowest (Figure 6B). The predicted immune subtypes significantly stratified patient survival (Log-rank test, p=0.001) (Figure 6C). C3 tumors demonstrated the best prognosis, followed by C2 and C1, while C4 had the worst prognosis. Interestingly, C2 tumors showed the highest cytolytic activity (Kruskal-Wallis, p=3.4e-9) and immune exhaustion (p=2.5e-12) (Figure 6D, S6A). This result implies that strong immune cytotoxicity might lead to exhaustion of the immune repertoire, compromising anti-tumor efficiency upon ICB treatment.
Figure 6. Expression Levels of HLA Genes Predict the Immune Subtypes and Stratify Patient Survival.
(A) Receiver Operating Characteristic (ROC) curve of a TCGA pan-cancer Random Forest classifier. Mean area under the curve (AUC) and standard deviations from cross-validation (10,000 repeats). (B) Expression of HLA I and II genes across the four immune subtypes predicted by the TCGA SKCM random forest classifier in the Liu anti-PD1 melanoma cohort. (C) Progression-free survival (PFS) by the predicted immune subtypes. P-value is calculated by the Log-rank test. (D) Immune exhaustion score (log-average expression of HAVCR2, TIGIT, LAG3, IDO1) across the predicted immune subtypes. P-value is calculated by the Kruskal-Wallis test.
Discussion
Based on the idea that antigen-presenting HLA class I and II molecules are fundamental for triggering anti-tumor immunity, we aimed to address how HLA expression shapes various immune microenvironments and tumor escape in a pan-cancer analysis from TCGA. While TMB is frequently used to predict immunogenicity and survival, HLA I and HLA II expression showed much stronger correlation with immune-related tumor characteristics. We reasoned that neoantigens generated in tumors with high TMB but low HLA expression could not be effectively presented to trigger immune activities. In recent years, neoantigen-based personalized cancer vaccines and adoptive cell therapy (ACT) are among the advanced immunotherapies to trigger neoantigen-specific T cell activity (45). Current in silico algorithms predict neoantigens based solely on a patient’s HLA types and tumor mutations (46,47). Here, we suggest that HLA expression is a crucial component that should be incorporated in neoantigen prediction algorithms.
We also considered HLA allelic diversity, which potentially facilitates the presentation of diverse neoantigens (43). However, unlike HLA expression levels, HLA allelic diversity did not show correlation with immune infiltration. One possible explanation is that high HLA expression despite low allelic diversity may still trigger immunity by presenting sufficient neoantigens even if the neoantigens are of low diversity. In contrast, high HLA allelic diversity, though having the potential to present a more diverse group of neoantigens, cannot compensate for low HLA expression in cells with reduced neoantigen presentation. Despite of its limited role in triggering immune activities, we found that high HLA allelic diversity in tumors expressing high levels of HLA genes correlated with better survival. This suggests that high HLA allelic diversity may contribute more to tumor elimination through the presentation of a diverse neoantigen pool when HLA expression is high.
While HLA I molecules expressed on tumors present endogenous neoantigens to CD8 cytotoxic T cells, most HLA II molecules expressed on professional APCs present exogenous neoepitopes to CD4 helper T cells (48). CD4 T cell priming is essential in activating CD8 cytotoxic T cells and forming prolonged memory (49,50). Alterations in multiple oncogenic pathways were correlated with HLA II downregulation. This suggests that regulation of HLA II expression in tumors might be negatively influenced by the aggressiveness of tumors with different oncogenic mutations, resulting in insufficient T cell priming. In addition, the contrasting impacts of HLA I and II molecules in MSI-H tumors on patient survival might be partially due to the different types of immune cells to which they present antigens. While increased presentation of antigens by HLA I molecules to CD8 T cells directly elicits cytotoxicity against the tumor cells, presentation by HLA II molecules to CD4 T cells may trigger systematic CD8 T cell activation, leading to less neoantigen selection and less tumor escape. Future work may elucidate the downstream mechanisms of HLA presentation in MSI-H tumors.
Strong immune selective pressure can lead to tumor immunoediting. Here, we considered DNA methylation of HLA I genes and HLA LOH as potential HLA-mediated immune evasion strategies. A subset of immunogenic tumors with high cytolytic activity harbored more hypermethylated HLA genes, potentially as a means to reduce neoantigen presentation and evade recognition. We suggest that HLA II upregulation may reduce HLA I hypermethylation-mediated immunoediting. Tumors with high HLA I fold change alone can experience strong T cell cytotoxicity in the absence of helper T cell activation, leading to immunoediting and eventually tumor escape.
HLA LOH correlated with varied survival outcomes across tissues. While previous studies propose that HLA I allelic loss results in tumors evading immune recognition potentially through loss of allele-specific expression (3,35), we did not observe any correlation between HLA LOH and HLA downregulation. This suggests that HLA LOH likely did not significantly reduce overall HLA I expression, but led to other downstream effects such as reduced presentation of the most immunogenic neoantigens on the cell surface due to allele-specific HLA loss. Furthermore, higher expression of HLA I genes could effectively compensate for the loss by allowing higher numbers of neoantigens to be presented, leading to tumor rejection and improved survival. Since overall HLA expression was not correlated with HLA LOH, this finding suggests a new way of rescuing the anti-tumor immune recognition in patients with HLA LOH. Although HLA LOH, unlike HLA gene methylation, is an irreversible hard lesion at the gene level, therapies that enhance the expression of HLA genes at the RNA and protein levels could be exploited. Possible future work includes inducing HLA gene expression with pro-inflammatory cytokines like IFN-γ in tumors with HLA LOH to evaluate if elevated gene expression rescues immune recognition and tumor elimination in vivo.
We further demonstrated the practicability of using HLA class I and class II gene expression to predict the immune subtypes of patients receiving anti-PD1 treatment. Immune infiltration and cytotoxicity are critical for tumor responsiveness to ICB (15). Interestingly, in spite of high immunogenicity, patients with C2 tumors did not have the best prognosis, likely due to immune exhaustion under strong selective pressure, resulting in inefficient anti-tumor immunity upon ICB. Machine learning models based on the HLA gene signature could potentially help oncologists diagnose the tumor immune microenvironment and predict patient prognosis in ICB-treated cohorts.
Our study has several limitations. First, the HLA expression metric we used was transcript-based and did not take into account post-translational modification, thus it might not accurately capture the final quantity of HLA molecules expressed on the cell surface. We also did not consider specific neoantigen pools in tumors that could bind to the HLA molecules and be presented to T cells. Nonetheless, our analysis of TCGA samples has emphasized the important role of HLA differential regulation in facilitating the formation of prolonged immune response in tumors with improved survival. We also demonstrate the power of HLA expression data in predicting various immune microenvironments. Our framework should be useful for cancer immunotherapy studies and for understanding immunoediting mechanisms.
Supplementary Material
Acknowledgments
NIH/NCI R01 CA121113 (R. Karchin).
The results published here are in part based upon data generated by the TCGA Research Network: https://www.cancer.gov/tcga. The visual overview was created with BioRender.com.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The Immune Landscape of Cancer. Immunity 2018;48(4):812–30 e14 doi 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, et al. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front Immunol 2019;10:168 doi 10.3389/fimmu.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol 2016;39:44–51 doi 10.1016/j.coi.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radwan J, Babik W, Kaufman J, Lenz TL, Winternitz J. Advances in the Evolutionary Understanding of MHC Polymorphism. Trends Genet 2020;36(4):298–311 doi 10.1016/j.tig.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol 2015;15(4):203–16 doi 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wieczorek M, Abualrous ET, Sticht J, Alvaro-Benito M, Stolzenberg S, Noe F, et al. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front Immunol 2017;8:292 doi 10.3389/fimmu.2017.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halenius A, Gerke C, Hengel H. Classical and non-classical MHC I molecule manipulation by human cytomegalovirus: so many targets-but how many arrows in the quiver? Cell Mol Immunol 2015;12(2):139–53 doi 10.1038/cmi.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellins ED, Stern LJ. HLA-DM and HLA-DO, key regulators of MHC-II processing and presentation. Curr Opin Immunol 2014;26:115–22 doi 10.1016/j.coi.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richman LP, Vonderheide RH, Rech AJ. Neoantigen Dissimilarity to the Self-Proteome Predicts Immunogenicity and Response to Immune Checkpoint Blockade. Cell Syst 2019;9(4):375–82 e4 doi 10.1016/j.cels.2019.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 2017;17(4):209–22 doi 10.1038/nrc.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roudko V, Greenbaum B, Bhardwaj N. Computational Prediction and Validation of Tumor-Associated Neoantigens. Front Immunol 2020;11:27 doi 10.3389/fimmu.2020.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akazawa Y, Nobuoka D, Takahashi M, Yoshikawa T, Shimomura M, Mizuno S, et al. Higher human lymphocyte antigen class I expression in early-stage cancer cells leads to high sensitivity for cytotoxic T lymphocytes. Cancer Sci 2019;110(6):1842–52 doi 10.1111/cas.14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo N, Nixon MJ, Gonzalez-Ericsson PI, Sanchez V, Opalenik SR, Li H, et al. DNA methyltransferase inhibition upregulates MHC-I to potentiate cytotoxic T lymphocyte responses in breast cancer. Nat Commun 2018;9(1):248 doi 10.1038/s41467-017-02630-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seliger B, Ruiz-Cabello F, Garrido F. Chapter 7 IFN Inducibility of Major Histocompatibility Antigens in Tumors. Advances in Cancer Research 2008. p 249–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D, Schilling B, Liu D, Sucker A, Livingstone E, Jerby-Arnon L, et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med 2019;25(12):1916–27 doi 10.1038/s41591-019-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014;30(7):923–30 doi 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 17.Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nature Communications 2013;4(1) doi 10.1038/ncomms3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szolek A, Schubert B, Mohr C, Sturm M, Feldhahn M, Kohlbacher O. OptiType: precision HLA typing from next-generation sequencing data. Bioinformatics 2014;30(23):3310–6 doi 10.1093/bioinformatics/btu548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawaguchi S, Higasa K, Shimizu M, Yamada R, Matsuda F. HLA-HD: An accurate HLA typing algorithm for next-generation sequencing data. Hum Mutat 2017;38(7):788–97 doi 10.1002/humu.23230. [DOI] [PubMed] [Google Scholar]
- 20.Ball MP, Li JB, Gao Y, Lee JH, LeProust EM, Park IH, et al. Targeted and genome-scale strategies reveal gene-body methylation signatures in human cells. Nat Biotechnol 2009;27(4):361–8 doi 10.1038/nbt.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018;173(2):321–37 e10 doi 10.1016/j.cell.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortes-Ciriano I, Lee S, Park WY, Kim TM, Park PJ. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun 2017;8:15180 doi 10.1038/ncomms15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beck AH, Espinosa I, Edris B, Li R, Montgomery K, Zhu S, et al. The macrophage colony-stimulating factor 1 response signature in breast carcinoma. Clin Cancer Res 2009;15(3):778–87 doi 10.1158/1078-0432.CCR-08-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabro A, Beissbarth T, Kuner R, Stojanov M, Benner A, Asslaber M, et al. Effects of infiltrating lymphocytes and estrogen receptor on gene expression and prognosis in breast cancer. Breast Cancer Res Treat 2009;116(1):69–77 doi 10.1007/s10549-008-0105-3. [DOI] [PubMed] [Google Scholar]
- 25.Teschendorff AE, Gomez S, Arenas A, El-Ashry D, Schmidt M, Gehrmann M, et al. Improved prognostic classification of breast cancer defined by antagonistic activation patterns of immune response pathway modules. BMC Cancer 2010;10:604 doi 10.1186/1471-2407-10-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf DM, Lenburg ME, Yau C, Boudreau A, van 't Veer LJ. Gene co-expression modules as clinically relevant hallmarks of breast cancer diversity. PLoS One 2014;9(2):e88309 doi 10.1371/journal.pone.0088309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol 2004;2(2):E7 doi 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, et al. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 2015;12(5):453–7 doi 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen M, Andreatta M. NetMHCpan-3.0; improved prediction of binding to MHC class I molecules integrating information from multiple receptor and peptide length datasets. Genome Med 2016;8(1):33 doi 10.1186/s13073-016-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015;160(1–2):48–61 doi 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierini F, Lenz TL. Divergent Allele Advantage at Human MHC Genes: Signatures of Past and Ongoing Selection. Mol Biol Evol 2018;35(9):2145–58 doi 10.1093/molbev/msy116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 2004;5:113 doi 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grantham R Amino Acid Difference Formula to Help Explain Protein Evolution. Science 1974;185(4154):3 doi 10.1126/science. [DOI] [PubMed] [Google Scholar]
- 34.Eisenberg E, Levanon EY. Human housekeeping genes, revisited. Trends Genet 2013;29(10):569–74 doi 10.1016/j.tig.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 35.McGranahan N, Rosenthal R, Hiley CT, Rowan AJ, Watkins TBK, Wilson GA, et al. Allele-Specific HLA Loss and Immune Escape in Lung Cancer Evolution. Cell 2017;171(6):1259–71 e11 doi 10.1016/j.cell.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell 2018;173(2):400–16 e11 doi 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carosella ED, Moreau P, Le Maoult J, Le Discorde M, Dausset J, Rouas-Freiss N. HLA-G Molecules: from Maternal–Fetal Tolerance to Tissue Acceptance. Advances in Immunology 2003. p 199–252. [DOI] [PubMed] [Google Scholar]
- 38.Poluektov YO, Kim A, Sadegh-Nasseri S. HLA-DO and Its Role in MHC Class II Antigen Presentation. Front Immunol 2013;4:260 doi 10.3389/fimmu.2013.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strickler JH, Hanks BA, Khasraw M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin Cancer Res 2021;27(5):1236–41 doi 10.1158/1078-0432.CCR-20-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin A, Zhang J, Luo P. Crosstalk Between the MSI Status and Tumor Microenvironment in Colorectal Cancer. Front Immunol 2020;11:2039 doi 10.3389/fimmu.2020.02039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doytchinova IA, Guan P, Flower DR. Identifiying human MHC supertypes using bioinformatic methods. J Immunol 2004;172(7):4314–23 doi 10.4049/jimmunol.172.7.4314. [DOI] [PubMed] [Google Scholar]
- 42.Sidney J, Peters B, Frahm N, Brander C, Sette A. HLA class I supertypes: a revised and updated classification. BMC Immunol 2008;9:1 doi 10.1186/1471-2172-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chowell D, Krishna C, Pierini F, Makarov V, Rizvi NA, Kuo F, et al. Evolutionary divergence of HLA class I genotype impacts efficacy of cancer immunotherapy. Nat Med 2019;25(11):1715–20 doi 10.1038/s41591-019-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maleno I, Lopez Nevot MA, Seliger B, Garrido F. Low frequency of HLA haplotype loss associated with loss of heterozygocity in chromosome region 6p21 in clear renal cell carcinomas. Int J Cancer 2004;109(4):636–8 doi 10.1002/ijc.20000. [DOI] [PubMed] [Google Scholar]
- 45.Blass E, Ott PA. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat Rev Clin Oncol 2021;18(4):215–29 doi 10.1038/s41571-020-00460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lauss M, Donia M, Harbst K, Andersen R, Mitra S, Rosengren F, et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat Commun 2017;8(1):1738 doi 10.1038/s41467-017-01460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto TN, Kishton RJ, Restifo NP. Developing neoantigen-targeted T cell-based treatments for solid tumors. Nat Med 2019;25(10):1488–99 doi 10.1038/s41591-019-0596-y. [DOI] [PubMed] [Google Scholar]
- 48.Axelrod ML, Cook RS, Johnson DB, Balko JM. Biological Consequences of MHC-II Expression by Tumor Cells in Cancer. Clin Cancer Res 2019;25(8):2392–402 doi 10.1158/1078-0432.CCR-18-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tay RE, Richardson EK, Toh HC. Revisiting the role of CD4(+) T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther 2021;28(1–2):5–17 doi 10.1038/s41417-020-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thibodeau J, Bourgeois-Daigneault MC, Lapointe R. Targeting the MHC Class II antigen presentation pathway in cancer immunotherapy. Oncoimmunology 2012;1(6):908–16 doi 10.4161/onci.21205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This paper analyzes existing, publicly available data. Gene expression data and downstream analysis data are available within the article and its supplementary data files.
All original code has been deposited at https://github.com/KarchinLab/HLA-Mediated-Tumor-Immunogenicity-Manuscript and is publicly available as of the date of publication.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.