Figure 3.
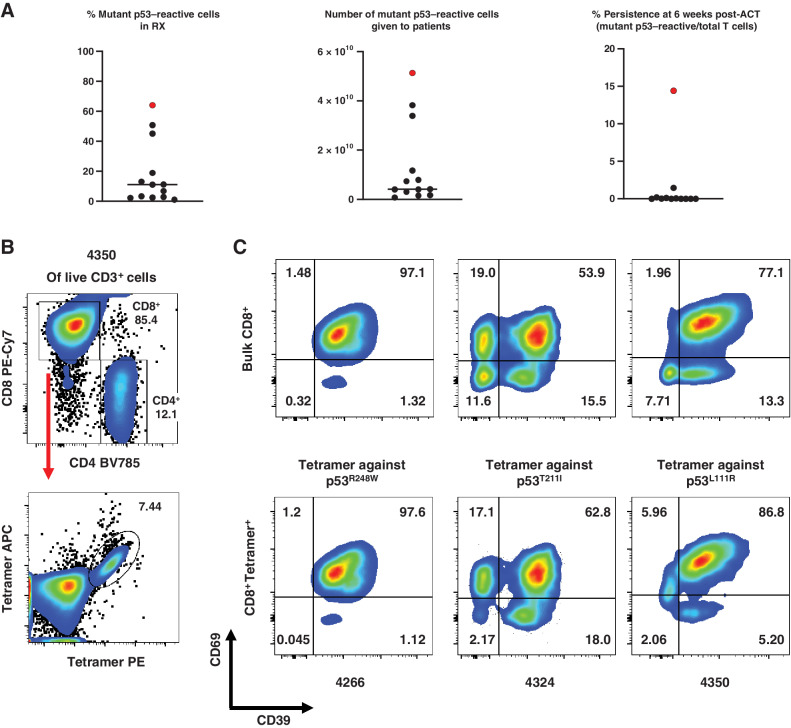
Autologous TIL ACT for the treatment of 12 patients with chemorefractory epithelial cancers. A, Characterization of the infusion products for the 12 autologous TIL ACTs. The ACT sample with genetically engineered PBL for patient 4349 is included for comparison and is marked in red. Bar denotes median. Detailed information is available in Table 2. RX, infusion product. B, Representative tetramer staining analysis by flow cytometry. Following positive gating of live CD3+ cells of patient 4350’s infusion product TILs, CD4, and CD8 gating (top) and tetramer staining of CD8+ cells (bottom) are presented. C, Phenotypic analysis of antigen-specific or bulk CD8+ T cells from the infusion products for patients 4266, 4324, and 4350 by flow cytometry. Expression of CD39 and CD69 in Bulk CD8+ T cells (CD3+CD8+; top) or tetramer-stained cells (CD3+CD8+Tetramer+; bottom) are shown. Due to sample limitations, A–C, were not independently repeated.