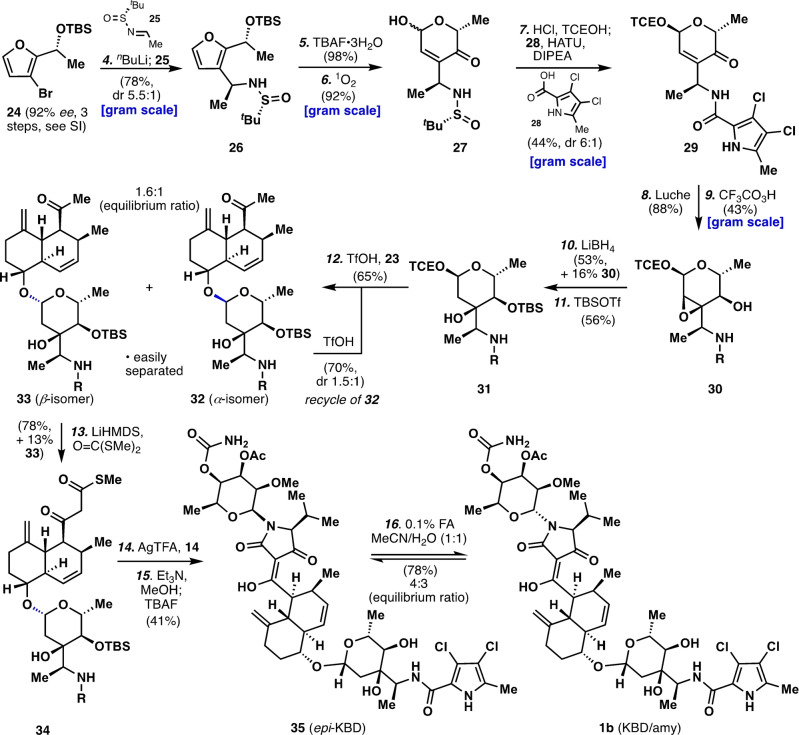
Scheme 2.
Total Synthesis of kibdelomycin (1 b). Reagents and conditions: For the synthesis of compound 24, see step 1–3 in Supporting Information; 4) n‐BuLi (1.1 equiv), Et2O, −40 °C, 1 h, then 25 (1.2 equiv), −78 °C to rt, 2 h, 78 % (d.r. 5.5 : 1); 5) TBAF⋅3H2O (2.0 equiv), THF, rt, 0.5 h, 98 %; 6) MB (0.0014 equiv), O2, DCM, −78 °C, 2.5 h, then Me2S (5.0 equiv), −78 °C to rt, 2 h, 92 %; 7) pTsOH (0.2 equiv), TCEOH, rt, 1.5 h, then HCl (2.0 equiv), rt, 1.5 h, then HATU (2.0 equiv), 28 (2.0 equiv), DIPEA (5.5 equiv), DMF, rt, 8 h, 44 % (d.r. 6 : 1); 8) NaBH4 (4.0 equiv), CeCl3⋅7H2O (0.4 equiv), MeOH, 0 °C, 20 min, 88 %; 9) CF3CO3H (1.36 equiv), DCM, −40 °C to rt, 2 h, 43 %; 10) LiBH4 (6.0 equiv), toluene, 60 °C, 3 h, 53 % S9+16 % 30; 11) TBSOTf (6.0 equiv), Et3N (10.0 equiv), DCE, 7 h, 56 %; 12) 23 (2.0 equiv), 4 A MS, TfOH (1.0 equiv), DCM, rt, 2.5 h, 65 % (1.6 : 1, β:α); 12′) 23 (2.0 equiv), TCEOH (1.0 equiv), 4 A MS, TfOH (2.0 equiv), DCM, rt, 2.5 h, 70 % (1.5 : 1, β:α); 13) LiHMDS (20.0 equiv), CO(SMe)2 (12.0 equiv), THF, −78 to 30 °C, 6.5 h, 78 % 34+13 % 33; 14) 14 (3.0 equiv), 4 A MS, AgTFA (5.0 equiv), THF, rt, 2 h; 15) Et3N (5.0 equiv), MeOH, rt, 10 min, then TBAF (8.0 equiv), THF, rt, 0.5 h, 41 % (2 steps); 16) 0.1 % HCO2H (4.4 equiv) in MeCN/H2O, rt, 24 h, 78 % (4 : 3, 35/1 b). Bu=butyl, MB=methylene blue, TCE=trichloroethyl, HATU=O‐(7‐azabenzotriazol‐1‐yl)‐N,N,N′,N′‐tetramethyluronium hexafluorophosphate, DIPEA=diisopropylethylamine, AgTFA=silver trifluoroacetate, Tf=trifluoromethanesulfonyl, DCE=dichloroethane, LiHMDS=lithium bis(trimethylsilyl)amide.