Figure 2: Fibroblast heterogeneity in mouse skin wound healing.
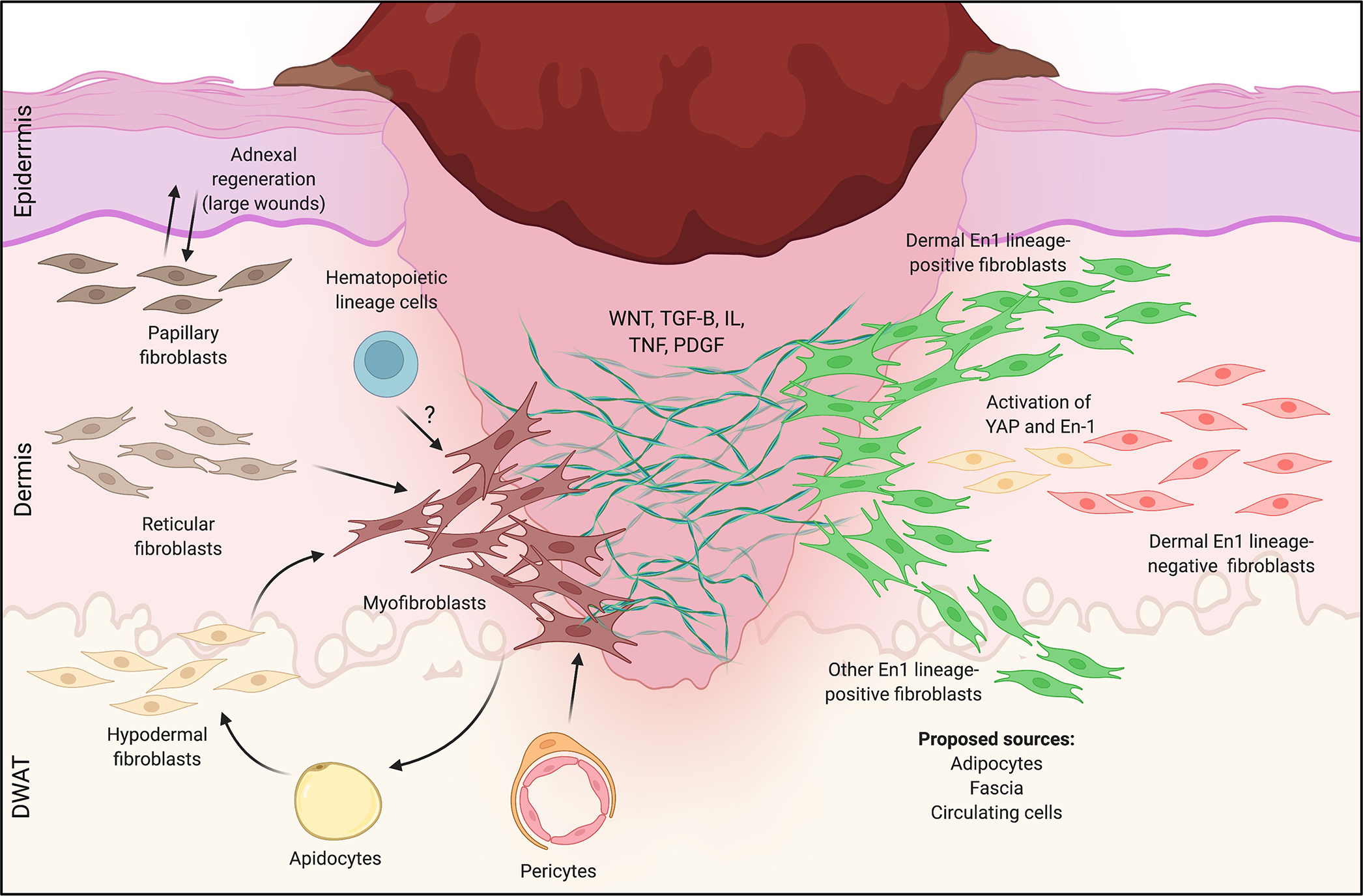
Left: Heterogeneous responses to wounding for the three microanatomically-defined lineages of fibroblasts (papillary, reticular, hypodermal). In small wounds, reticular (middle) and hypodermal (bottom) lineages dominate, giving rise to scarring myofibroblasts. In larger wounds (e.g., WIHN), papillary fibroblasts (top) preferentially interact with epidermal elements to drive sparse adnexal regeneration, and a subset of reticular layer-derived myofibroblasts regenerates adipocytes. Pericytes (bottom middle) and possibly circulating hematopoietic cells (“fibrocytes”, top middle) may also generate scarring myofibroblasts. Right: Heterogeneity in wound healing responses by Engrailed-1 (En-1) lineage-positive (green, EPF) and -negative (red, ENF) fibroblasts. EPFs are responsible for the vast majority of dorsal skin scarring in response to wounding, while ENFs do not contribute to fibrosis; a subset of ENFs reactivates En-1 to contribute postnatally-derived scarring EPFs. Proposed sources of scarring EPFs include the dermis (all layers), adipose tissue, circulating cells, and fascia. How these different lineages of fibroblasts (papillary, reticular, adipose, EPFs/ENFs, etc.) overlap and/or give rise to one another remains unclear.
