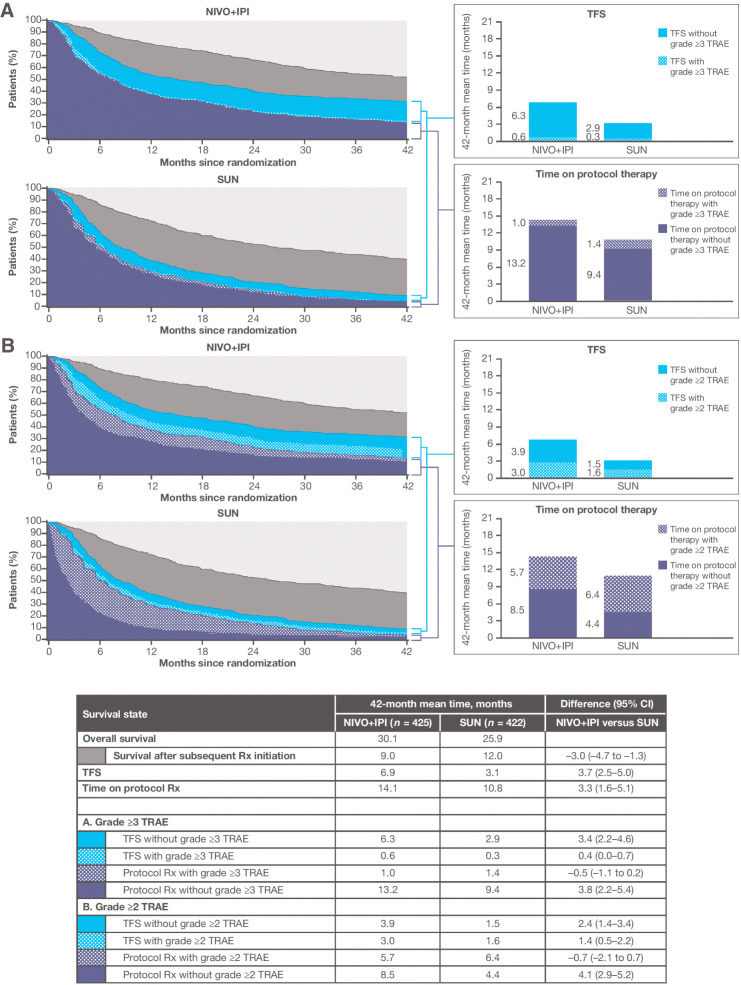
Figure 2.
Estimates of TFS, with and without toxicity, and other survival states over the 42-month period since randomization, according to treatment group, among 847 IMDC intermediate- and poor-risk patients. Toxicity is defined alternatively by grade ≥3 TRAEs (A) and grade ≥2 TRAEs (B). NIVO+IPI, nivolumab plus ipilimumab; Rx, therapy; SUN, sunitinib.