Fig. 2.
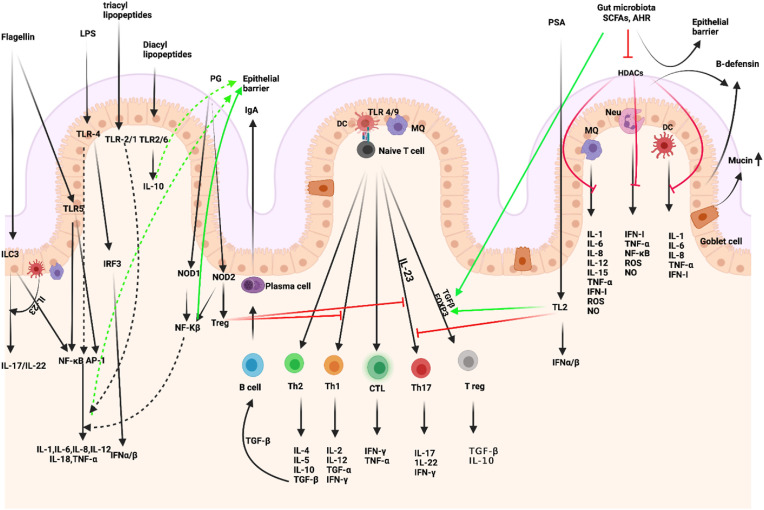
Intestinal microbiota-immunity interplay in immune homeostasis. Microbiome-derived TLR and NOD ligands and metabolites (e.g., SCFA, AHR ligands) act directly and indirectly. The effects of SCFAs include enhanced epithelial barrier function and immune tolerance, which promote gut homeostasis through specific mechanisms: 1. enhanced production of mucus by intestinal goblet cells, 2. inhibition of NF-κB; production of IL-18, 3. increased secretion of secretory IgA by B cells, 4. reduced expression of T cell-activating molecules on antigen-presenting cells, such as DCs, 5. and increased number and function of colonic Treg cells, including their expression of FOXP3 and their production of anti-inflammatory cytokines (TGF-β and IL-10). PSA is taken up by lamina propria DCs through a TLR2-dependent mechanism and presented to naïve CD4+ T cells. In the simultaneous presence of activated TGF-β, these cells can differentiate to Treg. IL-10 produced by these cells promotes immune homeostasis. Contrarily, IL-23 licensed through the same cascade promotes the expansion of pro-inflammatory Th17 cells. IECs-released factors such as retinoic acid (RA) and TGF-β, promote the development in the lamina propria of tolerogenic DCs that stimulate the differentiation of T cells into Treg. B cells differentiate into plasma cells (PCs) secreting IgA that translocate through the epithelium and are released into the mucus layer where control bacteria adhesion to host tissues. MQs, stimulated by signals such as flagellin, release IL-23, which in turn promotes the production of IL-22 by ILC3. (Figure has been created in biorender).