Abstract
BACKGROUND:
Coronavirus disease 2019 (COVID-19) presents mainly with mild symptoms and involvement of the respiratory system. Acute pancreatitis has also been reported during the course of COVID-19.
OBJECTIVE:
Our aim is to review and analyze all reported cases of COVID-19 associated acute pancreatitis, reporting the demographics, clinical characteristics, laboratory and imaging findings, comorbidities and outcomes.
DATA SOURCES:
We conducted a systematic search of Pubmed/MEDLINE, SciELO and Google Scholar to identify case reports and case series, reporting COVID-19 associated acute pancreatitis in adults.
STUDY SELECTION:
There were no ethnicity, gender or language restrictions. The following terms were searched in combination:“COVID-19” OR “SARS-CoV-2” OR “Coronavirus 19” AND “Pancreatic Inflammation” OR “Pancreatitis” OR “Pancreatic Injury” OR “Pancreatic Disease” OR “Pancreatic Damage”. Case reports and case series describing COVID-19 associated acute pancreatitis in adults were included. COVID-19 infection was established with testing of nasal and throat swabs using reverse transcription polymerase chain reaction. The diagnosis of acute pancreatitis was confirmed in accordance to the revised criteria of Atlanta classification of the Acute Pancreatitis Classification Working Group. Exclusion of other causes of acute pancreatitis was also required for the selection of the cases.
DATA EXTRACTION:
The following data were extracted from each report: the first author, year of publication, age of the patient, gender, gastrointestinal symptoms due to acute pancreatitis, respiratory-general symptoms, COVID-19 severity, underlying diseases, laboratory findings, imaging features and outcome.
DATA SYNTHESIS:
Finally, we identified and analyzed 31 articles (30 case reports and 1 case series of 2 cases), which included 32 cases of COVID-19 induced acute pancreatitis.
CONCLUSION:
COVID-19 associated acute pancreatitis affected mostly females. The median age of the patients was 53.5 years. Concerning laboratory findings, lipase and amylase were greater than three times the ULN while WBC counts and CRP were elevated in the most of the cases. The most frequent gastrointestinal, respiratory and general symptom was abdominal pain, dyspnea and fever, respectively. The most common imaging feature was acute interstitial edematous pancreatitis and the most frequent comorbidity was arterial hypertension while several patients had no medical history. The outcome was favorable despite the fact that most of the patients experienced severe and critical illness.
LIMITATIONS:
Our results are limited by the quality and extent of the data in the reports. More specifically, case series and case reports are unchecked, and while they can recommend hypotheses they are not able to confirm robust associations.
CONFLICT OF INTEREST:
None
INTRODUCTION
Acute pancreatitis is the leading cause of hospital admission for disorders of the gastrointestinal tract in several countries.1 Gallstones and alcohol overconsumption are well-established risk factors. Other factors, possibly genetic, probably have a role. Drugs are an additional causative factor of acute pancreatitis. Moreover, smoking and diabetes type II increase the probability of acute pancreatitis development.
Mild cases are generally successfully managed with a conservative approach. Severe cases frequently need admission to an intensive care unit for monitoring and managing complications of the disease, which are related to high rates of mortality, even when the treatment is optimal.2
Approximately 10% of cases of acute pancreatitis are considered to have infectious microorganisms as an underlying cause.3 These microorganisms include viruses (like Coxsackie B and hepatitis), bacteria (like Mycoplasma pneumonia and Leptospira), and parasites (like Ascaris lumbricoides and Fasciola hepatica). Each microorganism leads to acute pancreatitis through various mechanisms.3 Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread rapidly worldwide and is characterized by the World Health Organization as an international public health emergency. Besides typical symptoms and signs from respiratory system, acute pancreatitis has been reported during the course of the disease.4
COVID-19 associated pancreatic inflammation results from the expression of angiotensin converting enzyme 2 (ACE2) receptors in pancreatic tissue. The structural protein glycosylated-spike (S) protein, encoded by SARS-CoV-2 genome, primarily induces the immune response of the host. The S protein binds to ACE2 receptor sites on the cell surface membrane mediating the cell invasion. ACE2 receptors are not only expressed in lung alveolar type-2 cells. ACE2 receptors are expressed in the pancreas, in both exocrine glands and islets, in a higher grade than in the lungs.5,6 This expression of ACE2 receptors can lead to pancreatic cell damage during COVID-19 infection. Direct cytotoxic action of SARS-CoV-2 or indirect, immune-mediated, systemic inflammation could be the mechanism of pathogenesis for pancreatic injury.5,6
Globally, the incidence of acute pancreatitis ranges between 5 and 80 per 100 000 population, with the highest incidence observed in the United States and Finland.7 The incidence of SARS-CoV-2 infection varies among regions. Cyprus has the highest incidence of COVID-19 cases among its population in Europe at 55 424 per 100 000 people, followed by a rate of 52 738 per 100 000 in Iceland.8 In United States the incidence ranges between 2698 cases per 100 000 population in Hawaii and 14 541 cases per 100 000 population in North Dakota.8 In this study, we aimed to review and analyze all reported cases of COVID-19 associated acute pancreatitis, reporting the demographics, clinical characteristics, laboratory and imaging findings, comorbidities and outcomes.
CASES AND METHODS
Search strategy and article selection
We conducted a systematic search of Pubmed/MEDLINE, SciELO and Google Scholar to identify case reports and case series, reporting COVID-19 associated acute pancreatitis in adults, using the Patient, Intervention, Comparison and Outcome (PICO) Model.9 There were no ethnicity or gender restrictions. In addition, there were no language restrictions. We assessed all articles published from 01 January 2020 to 20 April 2021. A protocol of the study, including details of the methods used in the systematic review has been deposited in the PROSPERO database (https://www.crd.york.ac.uk/PROSPERO/) with the registration number CRD42021266917.
The following terms were searched in combination:“COVID-19” OR “SARS-CoV-2” OR “Coronavirus 19” AND “Pancreatic Inflammation” OR “Pancreatitis” OR “Pancreatic Injury” OR “Pancreatic Disease” OR “Pancreatic Damage”. The search was conducted by two reviewers (VEG, CD). Articles were first screened for relevance by title. Then they were evaluated by abstract. The relevant case reports were enrolled for full-text review. Moreover, a manual search of the lists of the references of these texts was performed for identifying additional relevant case reports and case series.
Case reports and case series describing COVID-19 associated acute pancreatitis in adults were included. COVID-19 infection was established with testing of nasal and throat swabs using reverse transcription polymerase chain reaction. The diagnosis of acute pancreatitis was confirmed in accordance to the revised criteria of the Atlanta Acute Pancreatitis Classification Working Group.10 At least two of the following three criteria had to be present for a diagnosis of acute pancreatitis: a) typical pain of acute pancreatitis (acute onset of a severe and persistent epigastric pain often with radiation to the back) b) serum lipase or amylase elevated at least three times the upper limit of normal; c) compatible imaging findings of acute pancreatitis on abdominal computed tomography (CT), on magnetic resonance imaging (MRI) or abdominal ultrasonography (U/S).10 Exclusion of other causes of acute pancreatitis was also required for the selection of the cases.
Data extraction
The following data were extracted from each report: the first author, year of publication, age of the patient, gender, gastrointestinal symptoms due to acute pancreatitis, respiratory-general symptoms, COVID-19 severity, underlying diseases, laboratory findings, imaging features and outcome. The tool suggested by Murad et al to assess the methodological quality and synthesis of the case series and case reports was utilized.11 The possible best score was 6 for a case report or a case series of good quality. The patients represented the whole experience of the researchers, the diagnosis of SARS-CoV-2 and the outcomes were adequately ascertained; other causes of pancreatitis were excluded. The follow-up was long enough for outcomes to occur and the described cases had sufficient details to allow other researchers to replicate the findings. Table 1 shows the use of the tool suggested by Murad et al in our review. In addition, we followed the PRISMA (Preferred Reporting Items For Systematic Reviews And Meta-Analyses) guidelines for writing this review.12
Table 1.
Tool for evaluating the methodological quality of case reports and case series of the current review suggested by Murad et al.11
| Domains | Leading explanatory questions | Cases and cases series included in the current review | Score |
|---|---|---|---|
| Selection | 1. Does the patient(s) represent(s) the whole experience of the investigator (center) or is the selection method unclear to the extent that other patients with similar presentation may not have been reported? | Yes | 1 |
| Ascertainment | 2. Was the exposure adequately ascertained? | Yes | 1 |
| 3. Was the outcome adequately ascertained? | Yes | 1 | |
| 4. Were other alternative causes that may explain the observation ruled out? | Yes | 1 | |
| Causality | 5. Was there a challenge/rechallenge phenomenon? | No | 0 |
| 6. Was there a dose-response effect? | No | 0 | |
| 7. Was follow-up long enough for outcomes to occur? | Yes | 1 | |
| Reporting | 8. Is the case(s) described with sufficient details to allow other investigators to replicate the research or to allow practitioners make inferences related to their own practice? | Yes | 1 |
| Total Score | 6 |
The statistical analysis of data was performed with IBM SPSS for Windows, Version 13.0 (Armonk, New York, United States: IBM Corp). Continuous variables were tested for normality of distribution by the Kolmogorov-Smirnov test. For normally distributed values, descriptive results are presented as mean (standard deviation) and median while categorical variables are mentioned as numbers and percentages. The meta-regression analysis was performed using a random-effects model and stepwise selection of variables.13 To determine if the findings affected the severity of COVID-19, we used a meta-regression analysis using the following equations: Severity1=ß0+ß1*log1(lipase) and Severity2=ß0+ß1*log2(amylase).
RESULTS
The systematic search identified 71 possibly relevant records after review of the title, abstract or full text screening, and after exclusion of duplicates (Figure 1). Forty records were excluded after careful screening of the titles and abstracts, since they did not mention COVID-19 associated with acute pancreatitis presented as case reports or case series. Finally, we identified 31 articles (30 case reports and 1 case series of 2 cases), which included 32 cases of COVID-19 induced acute pancreatitis (Tables 2, 3, 4).14-44 Nineteen patients were females (59.4%) and 13 patients were males (40.6%). The median age was 53.5 years (range 20-76 years). The median age of the females was 52 years (range 20-76 years) (median 52) years and the median age of the males was 48 (24-68) years.
Figure 1.
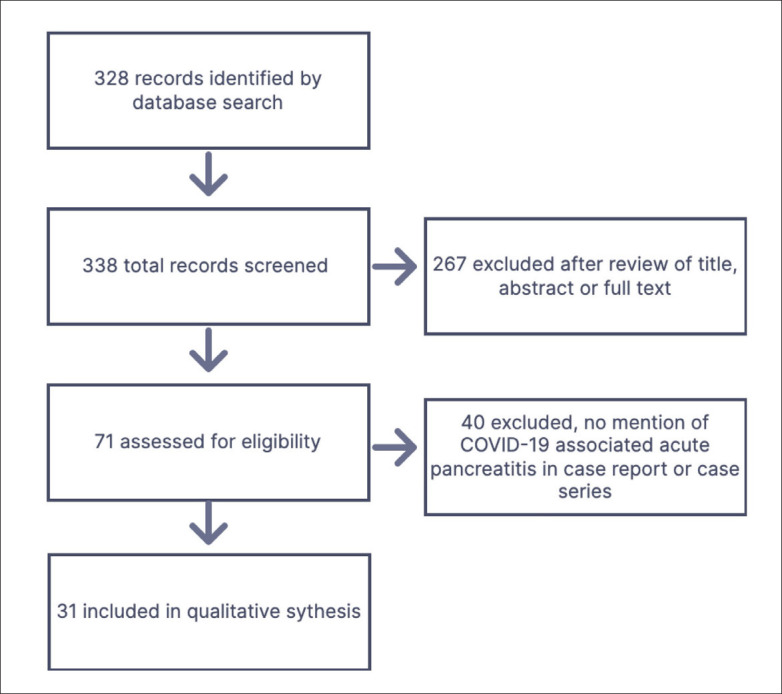
Flow diagram for study selection.
Table 2.
Demographic and clinical characteristics including COVID-19 severity and outcome of patients with COVID-19-induced acute pancreatitis.
| # | Author, Year | Age/Gender | Medical history | Gastrointestinal manifestations | Respiratory-general symptoms | Severity of COVID-19 | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Meyers, 202014 | 67/M | Arterial hypertension Cholecystectomy Alcohol use |
Abdominal pain | Dyspnea Fever |
Severe | Recovered |
| 2 | Karimzadeh, 202015 | 65/F | Arterial hypertension Asthma |
Abdominal pain Nausea |
Dyspnea Chills Myalgia |
Severe | Recovered |
| 3 | Shinohara, 202016 | 58/M | Arterial hypertension | Abdominal pain | Dyspnea Fever |
Critical | Recovered |
| 4 | Rabice, 202017 | 36/F | Pregnancy Diabetes mellitus Asthma Obesity |
Abdominal pain Nausea Vomiting |
Dry cough Fever Myalgia |
Severe | Recovered |
| 5 | Meireles, 202018 | 36/F | Post-HELLP syndrome Stage V chronic kidney disease Arterial hypertension |
Abdominal pain Nausea Vomiting |
Dry cough Dyspnea Fever |
Moderate | Recovered |
| 6 | Fernandes, 202019 | 36/F | No medical history | Abdominal pain | Dyspnea Fever Headache |
Moderate | Recovered |
| 7 | Alwaeli, 202020 | 30/M | No medical history | Abdominal pain Nausea Vomiting Diarrhea |
Dry cough Dyspnea Fever |
Critical | Recovered |
| 8 | Narang, 202021 | 20/F | Pregnancy Obesity Cholocystectomy |
Abdominal pain Nausea Vomiting |
Dyspnea | Critical | Recovered |
| 9 | Kandasamy, 202022 | 45/F | No medical history | Abdominal pain Nausea Vomiting |
Dyspnea | Severe | Recovered |
| 10 | Kumaran, 202023 | 67/F | Laparotomy and small bowel resection and anastomosis of superior mesenteric artery stenosis Arterial hypertension |
Abdominal pain Diarrhea Vomiting |
Dyspnea | Severe | Recovered |
| 11 | Acherjya, 202024 | 57/F | Arterial hypertension Diabetes mellitus Active malignancy of breast and larynx |
Abdominal pain Vomiting |
No respiratory symptoms Fever Generalized body ache Loss of smell Fatigue Arthralgia |
Severe | Recovered |
| 12 | Bokhari, 202025 | 32/M | No medical history | Abdominal pain Vomiting |
Productive cough Fever Myalgia |
Mild | Recovered |
| 13 | Mazrouei, 202026 | 24/M | N/A | Abdominal pain Nausea Vomiting |
No respiratory-other symptoms | Mild | Recovered |
| 14 | Patnaik, 202027 | 29/M | No medical history | Abdominal pain | Dyspnea Fever |
Moderate | Recovered |
| 15 | Schepis, 202028 | 67/F | Recent hospitalization for Interstitial Edematous acute pancreatitis of unknown origin |
Abdominal pain Vomiting |
No respiratory-other symptoms | Mild | Recovered |
| 16 | Aloysius, 202029 | 36/F | Chronic anxiety Obesity |
Abdominal pain Nausea Vomiting Diarrhea |
Dry cough Dyspnea Fever |
Critical | Recovered |
| 17 | Gonzalo-Voltas, 202030 | 76/F | Hypercholesterolemia Gastroesophageal reflux |
Abdominal pain Vomiting |
No respiratory-other symptoms | Mild | Recovered |
| 18 | Alves, 202031 | 56/F | Arterial hypertension | Abdominal pain | Dry cough Dyspnea Fatigue |
Critical | Recovered |
| 19 | Ghosh, 202032 | 63/M | Diabetes mellitus | No Gastrointestinal Symptoms | Dyspnea Dry cough Fever |
Severe | Recovered |
| 20 | Kataria, 202033 | 49/F | No medical history | Abdominal pain Nausea Vomiting |
Dyspnea Dry cough Lethargy Fever |
Critical | Recovered |
| 21 | Hadi, 202034 | 47/F | No medical history | Anorexia | Dyspnea Fever Headache Neck Pain Sore Throat |
Critical | N/A |
| 22 | Hadi, 202034 | 68/F | Arterial hypertension Hypothyroidism Osteoporosis |
Abdominal pain Vomiting Diarrhea |
Fever Fatigue Polydipsia |
Critical | N/A |
| 23 | Brikman, 202035 | 61/M | No medical history | Abdominal pain | Dyspnea Cough Fever |
Critical | Recovered |
| 24 | Lakshmanan, 202036 | 68/M | Nursing home resident Diabetes mellitus Arterial hypertension Stage IV chronic kidney disease |
Anorexia Nausea Vomiting |
No respiratory-other symptoms | Mild | Recovered |
| 25 | Miao, 202037 | 26/F | No medical history | Abdominal pain Vomiting |
No respiratory symptoms Fever | Mild | Recovered |
| 26 | Pinte, 202038 | 47/M | No medical history | Abdominal pain Nausea Constipation Lack of flatus |
Dry cough | Mild | Recovered |
| 27 | Anand, 202039 | 59/F | Cholecystectomy Thrombophilia |
Abdominal pain Constipation |
Cough Fever Sore Throat Myalgia |
Mild | Recovered |
| 28 | Wifi, 202140 | 72/F | Obesity Arterial hypertension Ischemic heart disease |
Abdominal pain Nausea Vomiting |
Cough Nasal Sneezing |
Severe | Recovered |
| 29 | Mohammadi Arbati, 202141 | 28/M | No medical history | Abdominal pain Nausea Vomiting |
Dyspnea Dry cough Fever Myalgia |
Critical | Recovered |
| 30 | Maalouf, 202142 | 62/M | Arterial hypertension Diabetes mellitus End-stage renal disease status Post Kidney Transplant |
Abdominal pain Diarrhea Vomiting Anorexia |
Dyspnea | Moderate | Recovered |
| 31 | AlHarm, 202143 | 52/F | Diabetes mellitus Arterial hypertension Hypothyroidism Obesity |
Abdominal pain Nausea Vomiting |
Dry cough Dyspnea Fever |
Severe | Recovered |
| 32 | Chivato Martín-Falquina, 202144 | 55/M | No medical history | No Gastrointestinal Symptoms | Dyspnea | Severe | Recovered |
HELLP: Hemolysis, Elevated Liver Enzymes, Low Platelet Count.
Table 3.
Laboratory and Imaging findings among the 32 cases following development of acute pancreatitis.
| # | Author, Year | Lipase (U/L) | Amylase (U/L) | WBC/CRP | Abdominal imaging features |
|---|---|---|---|---|---|
| 1 | Meyers, 202014 | >3 times of UNL | N/A | N/A/N/A | Abdominal CT: acute interstitial edematous pancreatitis. |
| 2 | Karimzadeh, 202015 | >3 times of UNL | <3 times of ULN | Normal/N/A | Abdominal CT: no abnormal findings. |
| 3 | Shinohara, 202016 | N/A | >3 times of UNL | Normal/Elevated | Abdominal CT: acute interstitial edematous pancreatitis. |
| 4 | Rabice, 202017 | >3 times of UNL | <3 times of ULN | Normal/N/A | Abdominal CT was not recommended as it would not change clinical management. |
| 5 | Meireles, 202018 | >3 times of UNL | >3 times of UNL | N/A/Elevated | Angio-abdominal CT: exclusion of ischemic changes |
| 6 | Fernandes, 202019 | >3 times of UNL | >3 times of UNL | N/A/N/A | Abdominal CT: acute interstitial edematous pancreatitis. |
| 7 | Alwaeli, 202020 | >3 times of UNL | <3 times of ULN | Normal/N/A | Abdominal CT: acute interstitial edematous pancreatitis. |
| 8 | Narang, 202021 | >3 times of UNL | >3 times of UNL | Elevated/N/A | Abdominal MRI: acute interstitial edematous pancreatitis. |
| 9 | Kandasamy, 202022 | >3 times of UNL | >3 times of UNL | Elevated/N/A | Abdominal CT: acute interstitial edematous pancreatitis. |
| 10 | Kumaran, 202023 | N/A | >3 times of UNL | Elevated/Elevated | Abdominal CT: necrotizing pancreatitis |
| 11 | Acherjya, 202024 | >3 times of UNL | <3 times of UNL | Decreased/Elevated | Abdominal CT: acute interstitial edematous pancreatitis. |
| 12 | Bokhari, 202025 | >3 times of UNL | >3 times of UNL | Elevated/Elevated | Abdominal CT: acute interstitial edematous pancreatitis. |
| 13 | Mazrouei, 202026 | >3 times of UNL | >3 times of UNL | N/A N/A | Abdominal CT: acute interstitial edematous pancreatitis. |
| 14 | Patnaik, 202027 | >3 times of UNL | >3 times of UNL | Elevated/Elevated | Abdominal CT, Abdominal U/S : acute interstitial edematous pancreatitis and no evidence of common bile duct calculi. |
| 15 | Schepis, 202028 | N/A | >3 times of UNL | Normal/N/A | Abdominal CT: large pancreatic pseudocyst causing a partial stomach outlet obstruction |
| 16 | Aloysius, 202029 | >3 times of UNL | >3 times of UNL | Normal/Elevated | Abdominal CT: normal gall bladder, biliary tract, with unremarkable pancreas. |
| 17 | Gonzalo-Voltas, 202030 | N/A | >3 times of UNL | Elevated/Elevated | Abdominal CT: acute interstitial edematous pancreatitis. |
| 18 | Alves, 202031 | >3 times of UNL | >3 times of UNL | N/A/N/A | Abdominal CT: acute interstitial edematous pancreatitis. |
| 19 | Ghosh, 202032 | <3 times of UNL | <3 times of UNL | Normal/Elevated | Abdominal CT: necrotizing pancreatitis |
| 20 | Kataria, 202033 | >3 times of UNL | >3 times of UNL | Normal/Elevated | Abdominal CT: acute interstitial edematous pancreatitis. |
| 21 | Hadi, 202034 | N/A | >3 times of UNL | Normal/Elevated | Abdominal U/S: acute interstitial edematous pancreatitis. |
| 22 | Hadi, 202034 | N/A | >3 times of UNL | Normal/Elevated | N/A |
| 23 | Brikman, 202035 | >3 times of UNL | <3 times of UNL | Elevated/N/A | Abdominal CT: acute interstitial edematous pancreatitis. |
| 24 | Lakshmanan, 202036 | >3 times of UNL | >3 times of UNL | Normal/Elevated | Abdominal CT: acute interstitial edematous pancreatitis (peripancreatic fat stranding, greatest around the tail, with mild duodenal wall thickening and adjacent fat stranding) |
| 25 | Miao, 202037 | >3 times of UNL | N/A | Normal/Elevated | Abdominal CT, abdominal U/S: acute interstitial edematous pancreatitis. |
| 26 | Pinte, 202038 | N/A | N/A | Elevated/Elevated | Abdominal CT: acute interstitial edematous pancreatitis. |
| 27 | Anand, 202039 | N/A | N/A | Elevated/Elevated | Abdominal CT: acute interstitial edematous pancreatitis. |
| 28 | Wifi, 202140 | >3 times of UNL | >3 times of UNL | Elevated/Elevated | Abdominal CT: without abnormal findings |
| 29 | Mohammadi Arbati, 202141 | >3 times of UNL | >3 times of UNL | Elevated/Normal | Abdominal CT: necrotizing pancreatitis |
| 30 | Maalouf, 202142 | >3 times of UNL | N/A | Decreased/Elevated | Abdominal MRI: necrotizing pancreatitis |
| 31 | AlHarm, 202143 | N/A | <3 times of UNL | Elevated/Normal | Abdominal CT: acute interstitial edematous pancreatitis. |
| 32 | Chivato Martín-Falquina, 202144 | N/A | >3 times of UNL | N/A N/A | Abdominal CT: acute interstitial edematous pancreatitis. |
CRP: C-reactive protein; CT: Computerized tomography; MRI: Magnetic resonance imaging, U/S: Ultrasonography, ULN: Upper limit of normal, WBC: White blood cells.
Table 4.
Demographic and clinical data from the 32 cases.
| Demographics | |
|---|---|
| Gender | |
| Males | 13/32 (59.4) |
| Females | 19/32 (40.6) |
| Gastrointestinal symptoms | |
| Abdominal pain | 28/32 (87.5) |
| Nausea | 14/32 (43.8) |
| Vomiting | 20/32 (62.5) |
| Diarrhea | 5/32 (15.6) |
| Constipation | 2/32 (6.3) |
| Anorexia | 3/32 (9.3) |
| Lack of flatus | 1/32 (3.1) |
| No gastrointestinal symptoms | 2/32 (6.3) |
| Respiratory/General symptoms | |
| Dyspnea | 20/32 (62.5) |
| Cough | 14/32 (43.8) |
| Fever | 19/32 (59.4) |
| Myalgia | 4/32 (12.5) |
| Fatigue | 3/32 (9.3) |
| Headache | 2/32 (6.3) |
| No respiratory or general symptoms | 4/32 (12.5) |
| No respiratory symptoms | 2/32 (6.3) |
| COVID-19 Severity | |
| Mild SARS-CoV-2 illness | 8/32 (25) |
| Moderate SARS-CoV-2 illness | 4/32 (12.5) |
| Severe SARS-CoV-2 illness | 10/32 (31.2) |
| Critical SARS-CoV-2 illness/Need for admission to ICU | 10/32 (31.2) |
| Laboratory findings following development of acute pancreatitis | |
| Amylase levels over three times of ULN | 20/27 (74.1) |
| Amylase levels less than three times of ULN | 7/27 (25.9) |
| Lipase levels over three times of ULN | 21/22 (95.5) |
| Lipase levels less than three times of ULN | 1/22 (0.5) |
| Elevated white blood cell count | 12/26 (46.2) |
| White blood cell count with normal limits | 12/26 (46.2) |
| Decreased WBC count | 2/26 (7.6) |
| Elevated levels of CRP | 18/20 (90) |
| C-reactive protein levels within normal limits | 2/20 (10) |
| Imaging features | |
| Acute interstitial edematous pancreatitis | 21/31 (67.8) |
| Necrotizing pancreatitis | 4/31 (12.9) |
| No abnormal imaging findings | 3/31 (9.7) |
| Abdominal CT was not performed due to pregnancy | 1/31 (3.2) |
| Angio-abdominal CT was conducted in order to exclude ischemic changes | 1/31 (3.2) |
| Large pancreatic pseudocyst causing a partial stomach outlet obstruction on abdominal CT | 1/31 (3.2) |
| Medical history | |
| Arterial hypertension | 11/31 (35.5) |
| Diabetes mellitus | 6/31 (19.4) |
| Obesity | 5/31 (16.1) |
| Cholecystectomy | 3/31 (9.7) |
| Asthma | 2/31 (6.5) |
| Chronic kidney disease | 3/31 (9.7) |
| Osteoporosis | 1/31 (3.2) |
| Hypothyroidism | 2/31 (6.5) |
| Gastroesophageal reflux | 1/31 (3.2) |
| Hypercholesterolemia | 1/31 (3.2) |
| Active cancer of larynx and breast | 1/31 (3.2) |
| Thrombophilia | 1/31 (3.2) |
| Pregnancy | 2/31 (6.5) |
| No medical history | 12/31 (38.5) |
| Outcomes | |
| Recovery | 30/30 (100) |
| Death | 0 |
Data are n (%); ULN: Upper limit of normal.
The majority of the patients had abdominal pain as clinical manifestation (28/32, 87.5%). Other gastrointestinal symptoms were nausea, vomiting, diarrhea, constipation, anorexia and lack of flatus, while 2 (6.3%) of the patients presented with no gastrointestinal symptoms. Twenty (62.5%) patients presented with dyspnea and 14 (43.8%) presented with cough. A majority of patients had fever (59.4%). Four (12.5%) patients had no respiratory or general symptoms, while 2 (6.3%) patients had no respiratory symptoms. According to classification into severity of illness categories by National Institutes of Health (NIH),45 8 (25%) patients had mild SARS-CoV-2 illness, 4 (12.5%) patients had moderate SARS-CoV-2 illness, 10 (31.2%) patients had severe SARS-CoV-2 illness and 10 (31.2%) patients had critical SARS-CoV-2 illness (Table 4).
The data on serum amylase levels in 27 patients was over three times the upper limit of normal (ULN), while the rest had amylase levels less than three times of ULN. Data about lipase levels were available in 22 patients. The majority of these patients (21/22, 95.5%) had lipase levels over three times of ULN while only 1 patient (1/22, 0.5%) had lipase levels less than three times of ULN. Data about white blood cells (WBC) count were available in 26 patients. Twelve patients (12/26, 46.2%) had elevated WBC count, 12 (46.2%) had WBC count with normal limits while 2 (7.6%) had decreased WBC count. Data on C-reactive protein were available in 20 patients. Eighteen patients (90%) had elevated levels of CRP while 2 (10%) had CRP levels within normal limits. Imaging data were available in 31 patients (Table 4). Twenty-one (67.8%) of the patients had abdominal CT or MRI features compatible with acute interstitial edematous pancreatitis. Medical history data were available in 31 patients. Arterial hypertension was most common, followed by diabetes mellitus, obesity, cholecystectomy and others. Two female patients were pregnant while 12 patients (38.5%) had no medical history.
Data on outcome were available in 30 cases. All these patients recovered (30/30, 100%). In two cases, the outcome was unknown because the article was published while patients were still hospitalized.
The meta-regression analysis included the 30 articles that presented full laboratory findings following development of acute pancreatitis (Table 5). The R value of 0.461 represents the simple correlation, which indicates a moderate degree of correlation. The R2 value indicates how much of the total variation in severity, the dependent variable, was explained by the independent variables. In this case, R2 indicated that only 21.3% could be explained by the independent variables. The association of the regression model was statistically significant (i.e., a good fit for the data) (P<.05) (Tables 6 and 7).
Table 5.
Data for the meta-regression meta-analysis.
| # | Author, Year | Age/Gender | Severity of COVID-19 | Lipase (U/L) | Amylase (U/L) |
|---|---|---|---|---|---|
| 1 | Meyers, 202014 | 67/M | Severe | >3 times of UNL | N/A |
| 2 | Karimzadeh, 202015 | 65/F | Severe | >3 times of UNL | <3 times of ULN |
| 3 | Shinohara, 202016 | 58/M | Critical | N/A | >3 times of UNL |
| 4 | Rabice, 202017 | 36/F | Severe | >3 times of UNL | <3 times of ULN |
| 5 | Meireles, 202018 | 36/F | Moderate | >3 times of UNL | >3 times of UNL |
| 6 | Fernandes, 202019 | 36/F | Moderate | >3 times of UNL | >3 times of UNL |
| 7 | Aiwaeli, 202020 | 30/M | Critical | >3 times of UNL | <3 times of ULN |
| 8 | Narang, 202021 | 20/F | Critical | >3 times of UNL | >3 times of UNL |
| 9 | Kandasamy, 202022 | 45/F | Severe | >3 times of UNL | >3 times of UNL |
| 10 | Kumaran, 202023 | 67/F | Severe | N/A | >3 times of UNL |
| 11 | Acherjya, 202024 | 57/F | Severe | >3 times of UNL | <3 times of UNL |
| 12 | Bokhari, 202025 | 32/M | Mild | >3 times of UNL | >3 times of UNL |
| 13 | Mazrouei, 202026 | 24/M | Mild | >3 times of UNL | >3 times of UNL |
| 14 | Patnaik, 202027 | 29/M | Moderate | >3 times of UNL | >3 times of UNL |
| 15 | Schepis, 202028 | 67/F | Mild | N/A | >3 times of UNL |
| 16 | Aloysius, 202029 | 36/F | Critical | >3 times of UNL | >3 times of UNL |
| 17 | Gonzalo-Voltas, 202030 | 76/F | Mild | N/A | >3 times of UNL |
| 18 | Alves, 202031 | 56/F | Critical | >3 times of UNL | >3 times of UNL |
| 19 | Ghosh, 202032 | 63/M | Severe | <3 times of UNL | <3 times of UNL |
| 20 | Kataria, 202033 | 49/F | Critical | >3 times of UNL | >3 times of UNL |
| 21 | Hadi, 202034 | 47/F | Critical | N/A | >3 times of UNL |
| 22 | Hadi, 202034 | 68/F | Critical | N/A | >3 times of UNL |
| 23 | Brikman, 202035 | 61/M | Critical | >3 times of UNL | <3 times of UNL |
| 24 | Lakshmanan, 202036 | 68/M | Mild | >3 times of UNL | >3 times of UNL |
| 25 | Miao, 202037 | 26/F | Mild | >3 times of UNL | N/A |
| 26 | Pinte, 202038 | 47/M | Mild | N/A | N/A |
| 27 | Anand, 202039 | 59/F | Mild | N/A | N/A |
| 28 | Wifi, 202140 | 72/F | Severe | >3 times of UNL | >3 times of UNL |
| 29 | Mohammadi Arbati, 202141 | 28/M | Critical | >3 times of UNL | >3 times of UNL |
| 30 | Maalouf, 202142 | 62/M | Moderate | >3 times of UNL | N/A |
| 31 | AlHarm, 202143 | 52/F | Severe | N/A | <3 times of UNL |
| 32 | Chivato Martin-Falquina, 202144 | 55/M | Severe | N/A | >3 times of UNL |
ULN: Upper limit of normal
Table 6.
Results of the regression analysis for lipase (n=24).
| Parameter | Beta estimate | z | P |
|---|---|---|---|
| ß0 | −.484 | −.780 | .442 |
| ß1 | .753 | 2.072 | .124 |
Severity1=−,484+753*log1(lipase)
Table 7.
Results of the regression analysis for amylase lipase (n=27).
| Parameter | Beta estimate | z | P |
|---|---|---|---|
| ß0 | −.484 | −.780 | .442 |
| ß1 | 1.223 | 1.586 | .042 |
Severity2=−,484+1,1223*log2(amylase)
DISCUSSION
There are very few case reports and case series describing COVID-19 induced acute pancreatitis. To our knowledge, we present the largest and most comprehensive systematic review of case reports and case series on SARS-CoV-2 infection causing acute pancreatitis. The ages of the patients were uniformly distributed with a median age of 53.5 years. The majority of the patients were females. Lipase and amylase were greater than three times the ULN while WBC counts and CRP were elevated in the most of the cases. The majority of the patients mentioned abdominal pain while other frequent symptoms were nausea and vomiting. The most common respiratory symptoms were dyspnea and cough. Fever was the most frequent general symptom and in some cases neither respiratory nor general symptoms were present. Most of the patients experienced severe and critical SARS-CoV-2 illness. The imaging features of abdominal CT were mostly compatible with acute interstitial edematous pancreatitis. The most frequent comorbidity was arterial hypertension and 38.5% of the patients had no medical history. In addition, where data were available, all the patients recovered.
The results of meta-regression analysis showed a low heterogeneity between the studies regarding the severity of COVID-19 disease and that serum levels of lipase and amylase had a moderate positive correlation with the severity of COVID-19 disease.
Data from studies about COVID-19 patients presenting with acute pancreatitis are limited. Szatmary et al in a study of hospitalized patients for acute pancreatitis found only 5 patients with SARS-CoV-2 infection in whom other causes of acute pancreatitis were excluded. All the patients were young adult males with a median age of 42 years and all were obese with no history of cardiovascular disease. There were no data about serum lipase levels; serum amylase levels were increased. Abdominal CT was used to establish the final diagnosis. The finding of pancreatic inflammation on CT was mild pancreatic edema without pancreatic or peripancreatic necrosis, compatible with acute interstitial edematous pancreatitis. In this study, all patients with COVID-19 associated acute pancreatitis recovered.46
Our systematic review was written after a comprehensive search of the literature with specific criteria for inclusion and quality assessment. However, our results are limited by the quality and extent of the data in the reports. More specifically, case series and case reports are unchecked, and while they can recommend hypotheses they are not able to confirm robust associations. Clinicians should be aware of the few cases reported in the literature, suggesting that acute pancreatitis can result from COVID-19. While case reports can provide signals, they are not strong enough for statistical inference. Thus, the evidence provided is insufficient to suggest systematic screening in patients with COVID-19 for pancreatic involvement, but should alert physicians of possible pancreatic involvement by SARS-CoV-2.
In conclusion, COVID-19 associated acute pancreatitis affected mostly females with a median age of 53.5 years. Concerning laboratory findings, lipase and amylase were greater than three times the ULN while WBC counts and CRP were elevated in the most of the cases. The most frequent gastrointestinal, respiratory and general symptom was abdominal pain, dyspnea and fever, respectively. The most common imaging feature was acute interstitial edematous pancreatitis and the most frequent comorbidity was arterial hypertension while several patients had no medical history. The outcome was favorable despite the fact that most of the patients experienced severe and critical illness. Our results warrant the need for larger controlled research to detect acute pancreatitis during COVID-19 course and to provide data on patient characteristics and outcomes.
Funding Statement
Funding: None.
REFERENCES
- 1.Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386(9988):85–96. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg JA, Hsu J, Bawazeer M, Marshall J, Friedrich JO, Nathens A, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg. 2016;59(2):128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawla P, Bandaru SS, Vellipuram AR. Review of Infectious Etiology of Acute Pancreatitis. Gastroenterology Res. 2017;10(3):153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta V. COVID-19 and Acute Pancreatitis: What Do Surgeons Need to Know? Indian J Surg. 2020:1–4. [DOI] [PMC free article] [PubMed]
- 5.Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, et al. Epidemiology, Genetic Recombination, and Pathogenesis of Coronaviruses. Trends Microbiol. 2016;24(6):490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, Long X, Zhang B, Chen X, Zhang Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin Gastroenterol Hepatol. 2020;18(9):2128–2130.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banks PA. Epidemiology, natural history, and predictors of disease outcome in acute and chronic pancreatitis. Gastrointest Endosc. 2002. Dec;56(6 Suppl):S226–30. [DOI] [PubMed] [Google Scholar]
- 8.Incidence of coronavirus (COVID-19) cases in Europe as of June 12, 2022, by country. 2022, Cited: July 23. Available from: https://www.statista.com/statistics/1110187/coronavirus-incidence-europe-by-country/
- 9.Systematic Reviews: What is a systematic review? [Internet]. Curtain University. Cited July 2022: https://libguides.library.curtin.edu.au/systematic-reviews [Google Scholar]
- 10.Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–11. [DOI] [PubMed] [Google Scholar]
- 11.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 14.Meyers MH, Main MJ, Obstein KL. A Case of COVID-19-Induced Acute Pancreatitis. Pancreas. 2020;49(10):e108–e109. [DOI] [PubMed] [Google Scholar]
- 15.Karimzadeh S, Manzuri A, Ebrahimi M, Huy NT. COVID-19 presenting as acute pancreatitis: Lessons from a patient in Iran. Pancreatology. 2020;20(5):1024–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinohara T, Otani A, Yamashita M, Wa-kimoto Y, Jubishi D, Okamoto K, et al. Acute Pancreatitis During COVID-19 Pneumonia. Pancreas. 2020;49(10):e106–e108. [DOI] [PubMed] [Google Scholar]
- 17.Rabice SR, Altshuler PC, Bovet C, Sullivan C, Gagnon AJ. COVID-19 infection presenting as pancreatitis in a pregnant woman: A case report. Case Rep Womens Health. 2020;27:e00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meireles PA, Bessa F, Gaspar P, Parreira I, Silva VD, Mota C, et al. Acalculous Acute Pancreatitis in a COVID-19 Patient. Eur J Case Rep Intern Med. 2020;7(6):001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes DA, Yumioka AS, Filho HRM. SARS-CoV-2 and acute pancreatitis: a new etiological agent? Rev Esp Enferm Dig. 2020;112(11):890. [DOI] [PubMed] [Google Scholar]
- 20.Alwaeli H, Shabbir M, Khamissi Sobi M, Alwaeli K. A Case of Severe Acute Pancreatitis Secondary to COVID-19 Infection in a 30-Year-Old Male Patient. Cureus. 2020;12(11):e11718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Narang K, Szymanski LM, Kane SV, Rose CH. Acute Pancreatitis in a Pregnant Patient With Coronavirus Disease 2019 (COVID-19). Obstet Gynecol. 2020;Publish Ahead of Print. [DOI] [PMC free article] [PubMed]
- 22.Kandasamy S. An unusual presentation of COVID-19: Acute pancreatitis. Ann Hepatobiliary Pancreat Surg. 2020;24(4):539–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumaran NK, Karmakar BK, Taylor OM. Coronavirus disease-19 (COVID-19) associated with acute necrotising pancreatitis (ANP). BMJ Case Rep. 2020;13(9):e237903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acherjya GK, Rahman MM, Islam MT, Alam AS, Tarafder K, Rahman MM, et al. Acute pancreatitis in a COVID-19 patient: An unusual presentation. Clin Case Rep. 2020;8(12):3400–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bokhari SMMA, Mahmood F. Case Report: Novel Coronavirus-A Potential Cause of Acute Pancreatitis? Am J Trop Med Hyg. 2020;103(3):1154–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazrouei SSA, Saeed GA, Al Helali AA. COVID-19-associated acute pancreatitis: a rare cause of acute abdomen. Radiol Case Rep. 2020;15(9):1601–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patnaik RNK, Gogia A, Kakar A. Acute pancreatic injury induced by COVID-19. ID-Cases. 2020;22:e00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schepis T, Larghi A, Papa A, Miele L, Panzuto F, De Biase L, et al. SARS-CoV2 RNA detection in a pancreatic pseudocyst sample. Pancreatology. 2020;20(5):1011–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aloysius MM, Thatti A, Gupta A, Sharma N, Bansal P, Goyal H. COVID-19 presenting as acute pancreatitis. Pancreatology. 2020;20(5):1026–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalo-Voltas A, Fernández-Pérez-Torres CU, Baena-Díez JM. Acute pancreatitis in a patient with COVID-19 infection. Med Clin (Barc). 2020;155(4):183–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alves AM, Yvamoto EY, Marzinotto MAN, Teixeira ACS, Carrilho FJ. SARS-CoV-2 leading to acute pancreatitis: an unusual presentation. Braz J Infect Dis. 2020;24(6):561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh A, Gupta V, Misra A. COVID19 induced acute pancreatitis and pancreatic necrosis in a patient with type 2 diabetes. Diabetes Metab Syndr. 2020;14(6):2097–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kataria S, Sharif A, Ur Rehman A, Ahmed Z, Hanan A. COVID-19 Induced Acute Pancreatitis: A Case Report and Literature Review. Cureus. 2020;12(7):e9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadi A, Werge M, Kristiansen KT, Pedersen UG, Karstensen JG, Novovic S, et al. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: Case report on three family members. Pancreatology. 2020;20(4):665–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brikman S, Denysova V, Menzal H, Dori G. Acute pancreatitis in a 61-year-old man with COVID-19. CMAJ. 2020;192(30):E858–E859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lakshmanan S, Malik A. Acute Pancreatitis in Mild COVID-19 Infection. Cureus. 2020;12(8):e9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao Y, Lidove O, Mauhin W. First case of acute pancreatitis related to SARS-CoV-2 infection. Br J Surg. 2020;107(8):e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinte L, Baicus C. Pancreatic involvement in SARS-CoV-2: case report and living review. J Gastrointestin Liver Dis. 2020;29(2):275–6. [DOI] [PubMed] [Google Scholar]
- 39.Anand ER, Major C, Pickering O, Nelson M. Acute pancreatitis in a COVID-19 patient. Br J Surg. 2020;107(7):e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wifi MN, Nabil A, Awad A, Eltatawy R. COVID-induced pancreatitis: case report. Egypt J Intern Med. 2021;33(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohammadi Arbati M, Molseghi MH. COVID-19 Presenting as Acute Necrotizing Pancreatitis. J Investig Med High Impact Case Rep. 2021;9:23247096211009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maalouf RG, Kozhaya K, El Zakhem A. SARS-CoV-2 induced necrotizing pancreatitis. Med Clin (Barc). 2021;S0025-7753(21)00025-7. [DOI] [PMC free article] [PubMed]
- 43.AlHarmi RAR, Fateel T, Sayed Adnan J, AlAwadhi K. Acute pancreatitis in a patient with COVID-19. BMJ Case Rep. 2021;14(2):e239656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chivato Martín-Falquina I, García-Morán S, Jiménez Moreno MA. Acute pancreatitis in SARS-CoV-2 infection. Beyond respiratory distress. Rev Esp Enferm Dig. 2021: Epub ahead of print. [DOI] [PubMed]
- 45.Clinical Spectrum of SARS-CoV-2 Infection. 2022, Cited: July 23 https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
- 46.Szatmary P, Arora A, Thomas Raraty MG, Joseph Dunne DF, Baron RD, Halloran CM. Emerging Phenotype of Severe Acute Respiratory Syndrome-Coronavirus 2-associated Pancreatitis. Gastroenterology. 2020. Oct;159(4):1551–4 [DOI] [PMC free article] [PubMed] [Google Scholar]


