Abstract
Background
The KMT2A gene, formerly named the MLL gene, is rearranged (KMT2Ar) in 70–75% of infants, 5–6% of children and 10–15% of adult patients with B cell acute lymphoblastic leukemia (B-ALL). The outcome after chemotherapy of pediatric cases remains poor, and only a few studies have investigated the clinical and laboratory features, treatment response and prognosis in Chinese populations.
Methods
A total of 48 B-ALL children with KMT2Ar were enrolled in the study, and clinical and laboratory data were collected and analyzed by age group. The relationship between prognosis and traditional risk factors and treatment response was investigated for these patients who received chemotherapy.
Results
The 48 enrolled patients included 28 males and 20 females; 18 (37.50%) or 30 (62.50%) patients were an age of < 12 m (infant B-ALL) or of > 12 m at onset. An initial WBC count of 300 × 109/L was detected in 7 (14.58%) patients; testicular leukemia (TL) or central nervous system involvement was found in 5 (10.41%) or 3 (6.25%) patients, respectively. Statistical differences were not found in the age groups of sex or initial WBC count, whereas TL was more common in the infant group (P < 0.05). 11q23 was detected in 18 patients; KMT2Ar was detected in 46 (95.83%) or 45 (93.75%) patients by FISH or multiplex RT–PCR technology, respectively; RNA-seq data were obtained for 18 patients, and 3 patients with uncommon KMT2Ar were identified. KMT2A-AFF1, KMT2A-MLLT3 and KMT2A-MLLT1 were the most common transcripts. Statistical differences were not found in treatment response by age groups, including dexamethasone induction, bone marrow (BM) smear status and minimal residual disease (MRD) level at different time points (TP), treatment-related mortality (TRM), or complete remission (CR) rate (P > 0.05); MRD levels monitored by FCM or PCR were unequal at the same TP. Four patients died of treatment, and TRM was 8.33%; 40 patients achieved CR, and the CR rate for the cohort was 83.33%. Seven patients quit, 15 patients relapsed, and the 5 yr cumulative relapse rate was 59.16 ± 9.16%; the 5 yr prospective EFS (pEFS) for patients who were included or excluded from the TRM group was 36.86 ± 8.48% or 40.84 ± 9.16%, respectively. Multivariate analysis for prognosis and hazard ratio was performed for 37 patients without TRM and revealed that an initial WBC count of > 300 × 109/L and a positive level of FCM-MRD were strongly related to a poor outcome for B-ALL patients with KMT2Ar (P < 0.05).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-022-09804-w.
Keywords: B cell acute lymphoblastic leukemia, KMT2A rearrangement, Pediatric, Treatment response, Minimal residual disease, Prognosis
Background
The lysine methyltransferase 2A (KMT2A) gene rearrangement, formerly known as the mixed lineage leukemia (MLL) gene or HRX rearrangement, occurs in 70–75% of infants, 5–6% of elderly children or 10–15% of adult patients with precursor B cell acute lymphoblastic leukemia (B-ALL) [1–3]. More than 90 partner genes for the KMT2A gene have been identified and 130 genes transcript had been found, and the KMT2A-AFF1 (MLL-AF4) transcript is the most common subtype. Transcripts of KMT2A rearrangements (KMT2Ar) are considered high-risk fusion genes in precursor B-ALL populations regardless of age, and long-term event-free survival (EFS) rates are less than 40–60%, even with intensive chemotherapy in developed or developing countries [4–6]. Allogeneic hematopoietic stem cell transplantation (AHSCT) provides more than 70–80% of the EFS rate in noninfant pediatric B-ALL patients with KMT2Ar at first complete remission (CR), but EFS of infant patients or ≥ CR2 patients or not CR patients remains poor [7, 8].
With the development of risk adaptive therapy, the prognosis of KMT2Ar B-ALL has improved, negative MRD levels at end of remission induction presented with 60.2% of 6 yr EFS of for infants’ group; therefore, it is important to distinguish these patients who did not benefit from chemotherapy at CR1. The study was designed to investigate the outcomes and prognostic factors of B-ALL children with KMT2Ar.
Patients and methods
Patients
Newly diagnosed pediatric B-ALL patients with KMT2Ar were enrolled in the study. These patients were admitted to the Children’s Hospital of Chongqing Medical University (CHCMU), Chengdu Women and Children Central Hospital (CWCCH), Caihong Hospital of Xianyang (CHX) and the Second Affiliated Hospital of Guizhou Medical University between January 2015 and December 2020. These patients were an age of < 18 yrs at diagnosis, and KMT2Ar was defined as at least one of t(?; 11q23) detected by chromosomal karyotype and/or fluorescence in situ hybridization (FISH), KMTA transcript identified by reverse-transcriptase polymerase chain (RT–PCR) and/or RNA sequencing (RNAseq) [1, 2, 9]. Exclusion criteria included patients with secondary leukemia, Burkitt leukemia (BL), mixed phenotype acute leukemia (MPAL), patients with coexisting specific fusion genes, and patients who had received chemotherapy before admission. Five patients were excluded, and 48 patients were enrolled in the study. The clinical, laboratory and prognostic data were collected and retrospectively analyzed. The study was approved by the Institutional Ethics Committee of CHCMU, and an informed consent form was signed by the guardians of these patients and/or the patients.
Enrolled patients received bone marrow (BM) aspiration and/or BM biopsy at initial diagnosis. The diagnosis of B-ALL was based on FAB classification and flow cytometry (FCM); Chromosomal karyotype was evaluated according to the International System of Human Cytogenetic Nomenclature of 2009 (ISCN-2009); interphase FISH of KMT2Ar, ETV6-RUNX1, BCR-ABL1, MYC and PDGFRb were performed as delineated in a literature report [9, 10]; common fusion genes, included KMT2Ar transcripts (KMT2A-AFF1, KMT2A-MLLT4, KMT2A-MLLT3, KMT2A-MLLT10, KMT2A-MLLT6, KMT2A-EPS15, KMT2A-MLLT11, KMT2A-FLNA, KMT2A-ELL, KMT2A-MLLT1 and dupKMT2A), were screened by multiplex nested RT–PCR (multiplex RT–PCR) [9–11]; Tumor DNA and/or total RNA samples of possible patients were obtained from BM samples at initial diagnosis, whole-exome sequencing (WES) and/or RNA sequencing (RNAseq) were performed by next-generation sequencing (NGS) as found in the literature and as we previously described [9, 10]. The positive KMT2Ar transcript results obtained by multiplex RT–PCR and/or RNA-seq were confirmed by split RT–PCR or quantitative real-time RT–PCR (qRT–PCR).
Treatment protocol and therapeutic evaluation
The patients were treated with the Chinese Children Cancer Group ALL 2015 protocol (CCCG-ALL-2015), and intrathecal injection (IT) for the prophylaxis of central nervous system leukemia (CNSL) was administered as the protocol required. The criteria for CNSL or testicular leukemia (TL) were defined as described in the literature. The treatment protocol was classified into induction, consolidation, intermediate treatment, reinduction and maintenance treatment [11, 12].
Complete blood counts (CBCs) were detected after four days of dexamethasone induction, and absolute blast cell counts were recorded. Blast cell counts < 1 × 109/L or ≥ 1 × 109/L were defined as good dexamethasone response (DGP) or poor dexamethasone response (DPR), respectively. BM studies were conducted at the following different time points (TPs): Day 19 of induction remission (TP1) and Day 46 of induction remission (TP2) or prior to consolidation (TP3). The remission status of BM smear samples was recorded as follows: M1 (CR, leukemic cells < 5%), M2 (leukemic cells 5–25%) and M3 (leukemic cells ≥ 25%). Minimal residual disease (MRD) level monitoring was performed by FCM and RT–PCR techniques; 10–4 was considered the limitation of FCM-MRD monitoring, and negative values were regarded as the cutoff values of PCR-MRD monitoring [9, 11].
These KMT2Ar B-ALL patients were considered the intermediate risk (IR) group, and the high risk (HR) group was classified as patients with ages of < 6 m and WBC counts of ≥ 300 × 109/L at initial diagnosis or an FCM-MRD level monitoring of ≥ 1% at TP2. Details of the protocol and the risk group classification are listed in the Supplementary Materials.
Statistical analysis
Complete remission (CR), BM relapse or extramedullary relapse were defined as diagnostic criteria, and EFS was defined as the time from diagnosis to the date of last contact for event-free survivors or to the first adverse event (not CR status after induction remission, relapse or refractory disease, death from any cause, secondary cancer or loss to follow-up). Following 31 January 2022, data on the clinical and laboratory findings, treatment responses, treatment-related mortality (TRM) and EFS of the enrolled patients were collected and retrospectively analyzed. SPSS 20.0 software (SPSS Inc., Chicago, IL) or GraphPad Prism 8.02 was used for statistical analysis and/or chart drawing. The impact of clinical and laboratory findings on EFS was assessed with the Kaplan–Meier method, and comparisons were made with the log-rank test. Multivariate analysis for prognosis and hazard ratio were performed using the Cox regression model. All probabilities reported were two-tailed, and P values of < 0.05 were regarded as significant differences.
Results
Baseline data
A total of 886 newly diagnosed B-ALL patients were enrolled the period, which included 857 non-infant patients (≥ 12 m) and 29 infant patients (< 12 m). 53 B-ALL patients with KMT2Ar were admitted in the period, 2 non-infant patients were eliminated owing to errors in immunophenotyping (1 patients was BAL, 1 patient diagnosed MPAL) KMT2Ar B-ALL were found in 65.51% (19 of 29) infant patients and in 3.73% (32/857) non-infant patients respectively. Chemotherapy was refused for 3 patients (1 infant patients and 2 non-infant patient), 48 consecutive patients were treated with the CCCG-ALL-2015 protocol and were enrolled in the study (Fig 1).
Fig. 1.
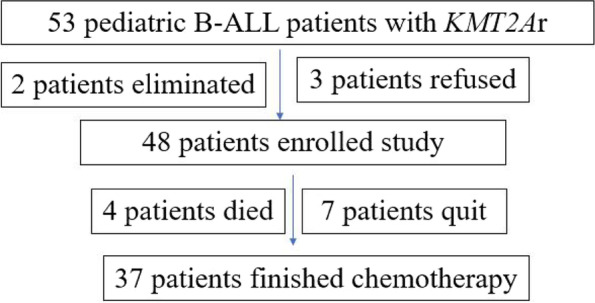
Patients enrolled the study
The clinical and laboratory findings of the 48 enrolled patients are listed in Table 1. The study group included 28 males and 20 females (1.53:1), and cancer family histories were found in 3 (6.25%) patients. The range, median or average values of age, white blood cell (WBC) count and lactate dehydrogenase (LDH) level at diagnosis were 3–173 m (18.50 m, 45.19 ± 7.41 m), 1.94–652.28 × 109/L (98.00, 149.79 ± 23.08 × 109/L) and 201–4059 U/L (874.50, 1005.90 ± 113.447 U/L), respectively. Initial WBC counts ≥ 50 × 109/L or 300 × 109/L were detected in 34 (70.83%) or 7 (14.58%) patients, respectively; LDH levels ≥ twofold of normal values (upper limit value: 330 U/L) were detected in 31 (64.58%) patients. Testicular or CNS involvement was found in 5 (10.41%) or 3 (6.25%) patients in the cohort.
Table 1.
Characteristics of the enrolled 46 patients
| Characteristics | Infant group (n = 18) | Non infant group (n = 30) | Fisher or t test | P = |
|---|---|---|---|---|
| Sex | ||||
| Male | 11 | 17 | > 0.9999 | |
| Female | 7 | 13 | ||
| WBC count | 153.49 ± 37.37 | 147.57 ± 29.82 | t = 0.1239, df = 36.80 | 0.9021 |
| ≥ 50 × 109/L | 14 | 20 | 0.5206 | |
| < 50 × 109/L | 4 | 10 | ||
| LDH (U/L) | 1114.13 ± 202.13 | 940.96 ± 136.44 | t = 0.7101, df = 32.11 | 0.4828 |
| ≥ 2 N | 13 | 18 | 0.5358 | |
| < 2 N | 5 | 12 | ||
| Extramedullary involvement | ||||
| Negative | 12 | 28 | 0.0396 | |
| Positive (CNSL, TL) | 6 (2,4) | 2 (1,1) | ||
| KMT2Ar distribution | ||||
| KMT2A-AFF1 | 10 | 14 | 0.7661 | |
| Non KMT2A-AFF1 | 8 | 16 | ||
Eighteen (37.50%) patients were < 12 m (infant B-ALL) at onset, and significant differences were not found in the age groups of sex or WBC count. The LDH level (P > 0.05) at initial diagnosis and extramedullary involvement (TL) were more common in the infant group (P < 0.05). The baseline data of the infant and noninfant groups in the cohort were similar (Table 1).
Chromosomal karyotypes were evaluated for 48 patients, and 8 patients were not evaluated. Normal karyotypes (46,XX/46,XY) were detected in 20 patients, and abnormal karyotypes were found in 20 patients. t(?;11q23) was detected in 18 patients, the detection rate of t(?;11q23) in the 40 analyzed patients was 45.00%. A DNA index of < 1.15 was not found in these 40 analytical patients. KMT2Ar was detectable in 46 (95.83%) or 45 (93.75%) patients by FISH or multiplex RT–PCR, respectively. RNA-seq data were obtained for 18 of the 48 patients. The same KMT2Ar transcript was obtained in 15 patients. Three patients with uncommon KMT2Ar transcripts were determined by RNA-seq. Two of the patients (KMT2A-CT45A1, KMT2A-CLTC) were not detected by multiplex RT–PCR, and 1 patient (KMT2A-USP2) was undetectable by both FISH and multiplex RT–PCR.
All 48 detectable cases of KMT2Ar transcript results were confirmed by split RT–PCR, which included 24 (50.00%) patients with KMT2A-AFF1 transcripts, 13 (27.08%) patients with KMT2A-MLLT3 transcripts, 5 (10.42%) patients with KMT2A-MLLT1 transcripts, and 1 (2.08%) patient with KMT2A-EPS15, KMT2A-MLLT10, KMT2A-FLNA, KMT2A-CLTC, KMT2A-CT45A1 or KMT2A-USP2 transcripts (Table 1).
Treatment response evaluation
Eighteen patients were classified as the infant group, blast cell counts at Day 5 of dexamethasone induction were evaluated, and negative or DGP were found in 6 or 11 cases, respectively. One patient died of sepsis before TP1, BM smears demonstrated 16 patients with M1 and 1 patient with M3; FCM-MRD level presented with < 0.01% or ≥ 0.01% in 6 or 11 patients, respectively, FCM-MRD level presented with < 1% or ≥ 1% was found in 12 or 5 patients, respectively; PCR-MRD level was determined, 6 patients were negative and 11 patients were positive. However, the results of FCM-MRD and PCR-MRD levels were not synchronized. Two patients died of sepsis and invasive fungal infections. BM samples were obtained from 15 patients at TP2, which showed that 12 patients were defined as CR by BM smear, and the CR rate was 66.66% in the total infant group. MRD levels were negative (< 0.01%) in 6 patients monitored by the FCM-MRD technique, and negative results were found in 7 patients by the PCR-MRD technique.
Thirty patients were defined as the noninfant group, and negative results or DGP were found in 14 or 18 patients, respectively. At TP1, BM smears demonstrated 28 patients with M1 and 2 patients with M2 or M3; FCM-MRD level presented with < 0.01% or ≥ 0.01% in 18 or 12 patients, respectively, FCM-MRD level presented with < 1% or ≥ 1% was found in 19 or 11 patients, respectively; negative or positive MRD results were found in 10 or 20 patients by PCR technique, FCM-MRD and PCR-MRD level were not synchronized. One patient died of sepsis, and BM samples were obtained from 29 patients at TP2. These 28 patients were regarded as CR by BM smear, and the CR rate was 93.33%. MRD levels were negative in 21 or 17 patients monitored by FCM or PCR, respectively.
Statistical differences were not found in the treatment response by age group, including dexamethasone induction, BM smear status and MRD level at different TPs, CR rates or TRMs (P > 0.05). The data are listed in Table 2. MRD levels monitored by FCM or PCR were unequal at the same TP, revealing that both FCM-MRD and PCR-MRD should be monitored for KMT2Ar B-All populations for treatment response evaluation.
Table 2.
Treatment response by age groups of the 48 enrolled patients
| Treat respond | Infant group (n = 18) | Non infant group (n = 30) | Fisher test | P = |
|---|---|---|---|---|
| dexamethasone induction (n = 48) | ||||
| Negative | 6 | 14 | 0.5461 | |
| Positive | 12 | 16 | ||
| DGR | 11 | 18 | > 0.9999 | |
| DPR | 7 | 12 | ||
| BM status at TP1a(n = 47) | ||||
| M1 | 16 | 28 | > 0.9999 | |
| M2 or M3 | 1 | 2 | ||
| FCM-MRD level at TP1a (n = 47) | ||||
| < 0.01% | 6 | 18 | 0.1351 | |
| ≥ 0.01% | 11 | 12 | ||
| < 1% | 12 | 19 | 0.7526 | |
| ≥ 1% | 5 | 11 | ||
| PCR-MRD level at TP1a (n = 47) | ||||
| Negative | 6 | 10 | > 0.9999 | |
| Positive | 11 | 20 | ||
| BM status at TP2a(n = 45) | ||||
| CR | 12 | 28 | 0.1073 | |
| Not CR | 3 | 1 | ||
| FCM-MRD level at TP2a (n = 45) | ||||
| < 0.01% | 6 | 21 | 0.0526 | |
| ≥ 0.01% | 9 | 8 | ||
| PCR-MRD level at TP2a (n = 45) | ||||
| Negative | 7 | 17 | 0.5213 | |
| Positive | 8 | 11 | ||
| Treat related mortality (n = 48) | ||||
| Yes | 3 | 1 | 0.1415 | |
| no | 15 | 29 | ||
a for survival patients
Analysis of prognosis and related risk factors
Of the 44 patients without TRM, 40 patients obtained CR status, and 4 patients did not achieve CR evaluated by BM smear. Seven (5 male and 2 female) of these 40 CR patients left the study because of family choice. Details regarding the patients that left the study are listed in Table 3. Chemotherapy was continued for 33 patients. After 31 January 2022, no further patients died of treatment, and 15 patients (BM relapsed: 14 patients; combination relapsed of BM and CNS: 1 patient) relapsed.
Table 3.
Basic data of these quit patients
| Patient | gender | Age (m) | WBC count (× 109/L) | KMT2Ar subtype | Extramedullary involvement | Blast cell at Day 5 (× 109/L) | D19 | D46 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BM smear | FCM-MRD (%) | PCR-MRD | BM smear | FCM-MRD (%) | PCR-MRD | |||||||
| 1 | M | 53 | 277.97 | KMT2A-AFF1 | N | 3.776 | CR | 6.21% | Negative | CR | 3.54% | Positive |
| 2 | M | 4 | 652.28 | KMT2A-MLLT1 | TL | 69.0477 | CR | 0.50% | Positive | CR | 0.00% | Positive |
| 3 | M | 145 | 595 | KMT2A-AFF1 | N | 0.8073 | CR | 1.73% | Positive | CR | 0.84% | Positive |
| 4 | F | 7 | 136.08 | KMT2A-AFF1 | N | 0 | CR | 0.00% | Negative | CR | 0.00% | Negative |
| 5 | M | 173 | 191.89 | KMT2A-AFF1 | TL | 0 | CR | 0.04% | Positive | CR | 0.00% | Positive |
| 6 | F | 26 | 23.71 | KMT2-MLLT3 | N | 1.0504 | CR | 9.05% | Positive | CR | 0.11% | Positive |
| 7 | M | 12 | 219.52 | KMT2A-AFF1 | N | 36.7445 | CR | 0.52% | Positive | CR | 0.44% | Positive |
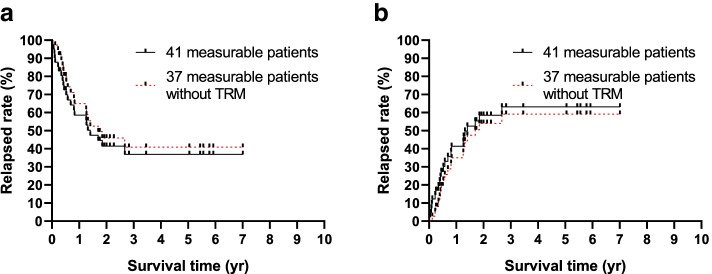
In general, 48 patients were enrolled the study, 4 patients died of treatment and TRM was 8.33%; 40 patients got CR and CR rate for the total 48 patients was 83.33%. 7 patients quit the study. Median follow up for the 37 patients without TRM was 15.48 m, 15 patients relapsed and 5 yr cumulative relapse rate for the 37 patients was 59.16 ± 9.16%; 5 yr prospective EFS (pEFS) for patients included TRM (41 patients) or excluded TRM (37 patients) was 36.86 ± 8.48% or 40.84 ± 9.16% respectively (Fig 2).
Fig. 2.
Survival of the cohort
Two of the 4 patients without CR underwent reinduction by hyper CVAD-based chemotherapy and achieved CR evaluated by BM smear. MRD levels were monitored by both FCM and PCR techniques. One patient achieved negative results for both FCM and PCR-MRD levels, and 1 patient presented with negative FCM-MRD levels and positive PCR-MRD levels. Five of 14 relapsed patients received reinduction, and CR status was achieved in 1 patient. CD19 CAR-T therapy was performed for 2 of the 4 patients, and both patients achieved CR evaluated by BM smear, FCM and PCR-MRD.
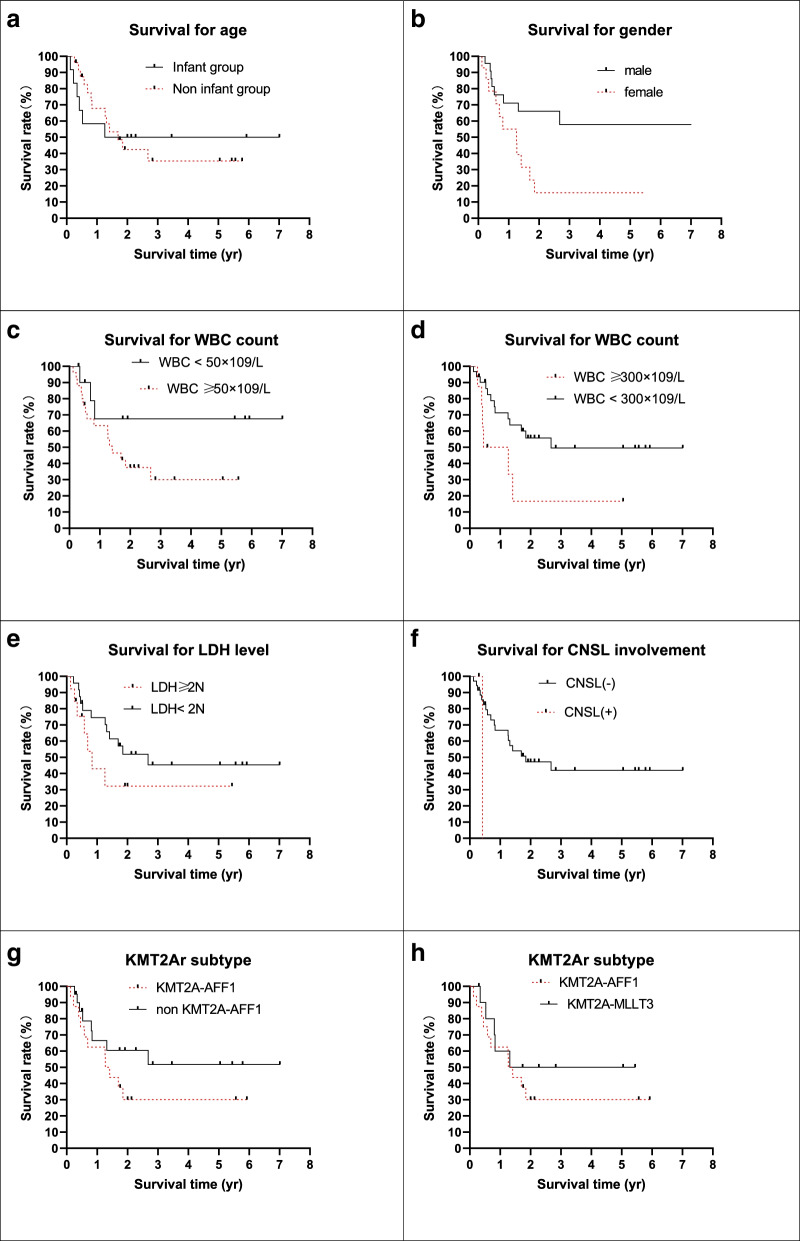
Hazard factors for the study were collected and analyzed for the 37 measurable patients without TRM. Traditional risk factors are listed in Table 4 and Fig. 3. The 5-year pEFS was longer in the male group, with WBC counts ≥ 50 × 109/L and 300 × 109/L (P < 0.05). Significant differences were not found in the different age and initial LDH level groups (P > 0.05). The CNSL data were too small to be compared. Subtypes of KMT2Ar were also evaluated, and the prognosis of the KMT2A-AFF1 transcript was lower than that of the other subtypes, but the difference was not statistically significant (P > 0.05).
Table 4.
Traditional risk factors and outcomes
| Demographics | n = 37a | % | pEFS | P = |
|---|---|---|---|---|
| Age group | ||||
| Infant group | 12 | 50.00 ± 14.43% | 0.9974 | |
| Noninfant group | 25 | 35.35 ± 11.28% | ||
| Gender | ||||
| Male | 23 | 57.80 ± 12.00% | 0.0160 | |
| Female | 14 | 15.71 ± 10.18% | ||
| WBC count | ||||
| ≥ 50 × 109/L | 25 | 30.03 ± 10.44% | 0.1178 | |
| < 50 × 109/L | 12 | 67.50 ± 15.51% | ||
| ≥ 300 × 109/L | 6 | 16.67 ± 14.83% | 0.0342 | |
| < 300 × 109/L | 31 | 49.57 ± 10.38% | ||
| LDH (U/L) | ||||
| ≥ 2 N | 24 | 32.23 ± 15.06% | 0.1762 | |
| < 2 N | 13 | 45.36 ± 11.07% | ||
| CNSL involvement | ||||
| CNSL- | 35 | 41.95 ± 9.34% | - | |
| CNSL + | 2 | - | ||
| KMT2Ar subtype | ||||
| KMT2A-AFF1 | 16 | 30.00 ± 11.78% | 0.1745 | |
| Non KMT2A-AFF1 | 21 | 51.83 ± 12.88% | ||
| KMT2A-AFF1 | 16 | 30.00 ± 11.78% | 0.4154 | |
| KMT2A-MLLT3 | 11 | 50.00 ± 15.81% | ||
a patients without TRM
Fig. 3.
Survival of the measurable patients by traditional risk factors
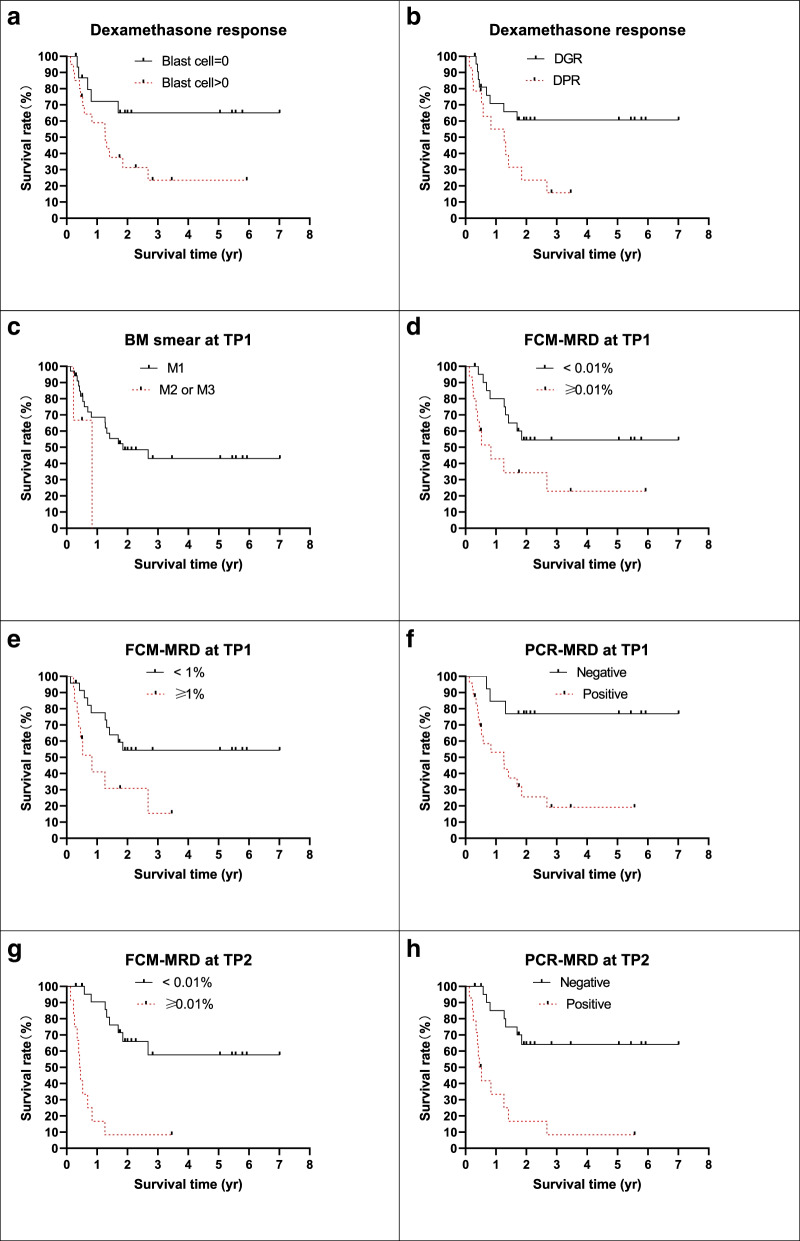
The relationship between treatment response and prognosis was also analyzed, and good treatment responses, including dexamethasone induction, BM smear at TP1, FCM-MRD or PCR-MRD level at TP1 and TP2, were related to better prognosis (Table 5, Fig 4). It should be noted that positive FCM-MRD results or positive PCR-MRD results were both strongly related to worse outcomes, and 5-year pEFS was < 10% when patients presented with positive MRD levels at TP2.
Table 5.
Treatment response and outcomes
| Demographics | n = 37a | % | pEFS | P = |
|---|---|---|---|---|
| Dexamethasone response | ||||
| Negative | 17 | 65.00 ± 12.68% | 0.0442 | |
| Positive | 20 | 23.44 ± 10.61% | ||
| DGR | 23 | 60.71 ± 10.87% | 0.0269 | |
| DPR | 14 | 15.71 ± 10.18% | ||
| BM status at TP1 | ||||
| M1 | 34 | 43.09 ± 9.52% | - | |
| M2 or M3 | 3 | - | ||
| FCM-MRD level at TP1 | ||||
| < 0.01% | 22 | % | 55.55 ± 11.24% | 0.0221 |
| ≥ 0.01% | 15 | % | 22.86 ± 12.90% | |
| < 1% | 24 | % | 52.38 ± 10.70% | 0.0175 |
| ≥ 1% | 13 | % | 15.39 ± 13.01% | |
| PCR-MRD level at TP1 | ||||
| Negative | 13 | % | 76.92 ± 11.69% | 0.0031 |
| Positive | 24 | % | 19.13 ± 9.43% | |
| FCM-MRD level at TP2 | ||||
| < 0.01% | 25 | % | 57.69 ± 12.01% | < 0.0001 |
| ≥ 0.01% | 12 | % | 8.33 ± 7.98% | |
| PCR-MRD level at TP2 | ||||
| Negative | 23 | % | 64.17 ± 10.93% | < 0.0001 |
| Positive | 14 | % | 8.33 ± 7.93% | |
a patients without TRM
Fig. 4.
Survival of the measurable patients by treatment respond
Multivariate analysis for prognosis and hazard ratio was performed using the Cox regression model (Table 6), which revealed that the female sex, an initial WBC count of > 300 × 109/L and a positive level of FCM-MRD were related to poor outcome for B-ALL patients with KMT2Ar; however, 5 males and 2 females achieved CR but quit chemotherapy, and larger samples and multicenter studies are needed to confirm this hypothesis. It seems that an initial WBC count > 300 × 109/L and FCM-MRD level were the key risk factors in pediatric B-ALL patients with KMT2Ar.
Table 6.
Multivariate analysis of prognoses and hazard ratios by the Cox regression model
| Multivariate analysis of prognoses and hazard ratios by the Cox regression model | ||||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | Wald | df | Sig | Exp(B) | 95.0% CI | ||
| Gender | Male vs. female | 1.218 | 0.500 | 5.931 | 1 | 0.015 | 3.380 | 1.268–9.008 |
| Age | Infant vs. non infant | 1.256 | NA | NA | 1 | 0.262 | NA | NA |
| WBC count (× 109/L) | > 300 vs. < 300 | -1.465 | 0.592 | 6.136 | 1 | 0.013 | 0.231 | 0.072–0.736 |
| Dexamethasone induction | DGR vs. DPR | 1.203 | NA | NA | 1 | 0.314 | NA | NA |
| TP1 FCM-MRD | Pos. vs. Neg | 0.080 | NA | NA | 1 | 0.778 | NA | NA |
| TP1 PCR-MRD | Pos. vs. Neg | 1.254 | NA | NA | 1 | 0.263 | NA | NA |
| TP2 FCM-MRD | Pos. vs. Neg | -1.465 | 0.592 | 6.136 | 1 | 0.013 | 0.231 | 0.072–0.328 |
| TP2 PCR-MRD | Pos. vs. Neg | 1.847 | NA | NA | 1 | 0.174 | NA | NA |
Discussion
The KMT2A gene is located at 11q23.3 and coexists with its partner genes. KMT2Ar encodes its relevant KMT2A protein and acts as an epigenetic regulator of transcriptional initiation through histone-3 lysine-4 methylation of target gene promoter regions, regulators of hematopoietic cell proliferation and differentiation and the homeobox A gene cluster. Deregulation of these genes initiates the development of leukemia through inhibition of proper hematopoietic development [13, 14]. The KMT2Ar fusion genes with their breakpoints code for their related proteins and are regarded as poor factors for both B-ALL and acute myeloid leukemia (AML) [13–15].
Pediatric or adult B-ALL with t(v;11q23.3) was regarded as a specific entity of recurrent genetic abnormalities in B-ALL by the WHO-2016 Classification of Hematopoietic and Lymphoid Tissues Tumors [14, 15]. KMT2Ar accounts for 70% of infant ALL cases, whereas it is not common in children older than 1 year [1, 2]. With different partner genes, more than 130 fusion gene transcripts have been identified; t(4;11)(q21;q23) and its transcript KMT2A-AFF1 (formerly named MLL-AF4 fusion gene) and t(9)(11)(p22;q23) and its transcript KMT2-MLLT3 (MLL-AF9) remain the most common subtype in both infant and elderly pediatric patients [1, 8, 16]. Our data also presented with these findings. Uncommon KMT2Ar transcripts were determined in 3 patients in the cohort by RNAseq, and we believe that KMT2A-CT45A1, KMT2A-CLTCit and KMT2A-USP2 transcripts were the 1st reported in the Chinese population. It also revealed that the RNA-seq technique was more sensitive than the FISH or the PCR technique for transfusion detection, i.e., rare KMT2Ar transcripts.
In our study, 4 patients died of severe infection, and the TRM was 8.33%. The data were higher than those of developed countries, partially due to the socioeconomic conditions in developing countries [6, 8–10, 14]. A total of 4 patients, including 3 of 18 infants or 1 of 30 elderly patients, died of TRM in the course of induction remission, which indicated that intensive care, antimicrobial prophylaxis and positive pressure rooms are important for neutropenic conditions.
The baseline data of the infant and noninfant groups were similar except that TL was more common in the infant group, and multiple centers and large data are needed to identify TL. The treatment response of the enrolled patients was evaluated and classified by age group. Statistical differences were not found between infant and noninfant patients, including dexamethasone induction response, BM smear, and MRD level determined by FCM or PCR at different TPs. A literature review showed that infant patients achieved a better treatment response (FCM-MRD level) when treated by the Interfant-06 protocol [17].
KMT2Ar B-ALL has a poor treatment response and prognosis. The CR rate in our cohort was 83.33%, and the 5-year pEFS for patients who did not have TRM was 40.84 ± 9.16%. These data were far lower than those of patients with B-ALL with good indicators, ETV6-RUNX1 transcripts or hyperploid karyotypes (> 95%, > 80–90%) [6, 7, 10].
The literature showed that an initial WBC count > 300 × 109/L was related to poor outcome [17], and our data also supports this hypothesis. EFS was longer in males than in females in the cohort, but the sample size was too small to confirm it. KMT2Ar is a reverse prognostic factor for B-ALL regardless of different KMT2Ar subtypes in our study. The result was different from those in literature reports, and we think it was partly due to different treatment strategies [1, 18, 19]. Further studies are needed to confirm this.
Treatment response was a strong indicator for KMT2Ar B-ALL patients. Patients who were sensitive to dexamethasone induction presented with prolonged 5 yr pEFS compared with these patients who were not. Similar results were identified in early studies for prednisone induction [6, 10], revealing that the treatment response to glucocorticoids remains a helpful method for predicting the prognosis of pediatric B-ALL with KMT2Ar.
In our study, pEFS for patients with positive MRD level at TP1 for FCM (cutoff value < 0.01%) or RT-PCR were < 20 -30%; whereas pEFS for patients with positive MRD level at TP2 were < 10–15%. Multivariable analysis for prognosis and hazard ratio was performed, it reveals that initial WBC count > 300 × 109/L or positive FCM-MRD level at TP2 was related to poor outcome. MRD level monitored by FCM and/ or RT-PCR were good predictor of outcome for pediatric B-ALL patients at any time points [17, 18], sensitivity and specificity for MRD monitored by FCM or RT-PCR at different TP remained different, further studied were need for the values for prognosis prediction in these patients. Infant patients treated with Interfant-06 protocol and presented with negative MRD level obtained with 6 yr EFS of 68.2% (95% CI, 55.2 to 78.1) [17]; 2 patients without CR in our study underwent re-induction by hyper CVAD based chemotherapy and achieved CR. These data prompts that Interfant-06 protocol might be more suitable for infant B-ALL patients with KMT2Ar [9, 19], we hypothesis that outcome of KMT2Ar positive B-ALL patients might be improved by protocol contain high dosage of cytarabine, further experimental and clinical studies were need. Our data reveal that prognosis of patients with poor treatment respond or initial WBC count > 300 × 109/L remained poor, literatures reports showed that B-ALL patients with KMT2Ar may benefit from allo-HSCT [7], these patients should receive allo-HSCT at CR1.
In conclusion, we assessed the outcomes and risk factors for Chinese pediatric B-ALL patients with KMT2Ar, outcomes remained poor by conventional chemotherapy. Allo-HSCT at CR1 for patients with risk factors might improve its prognosis; effects of high dose cytarabine had been identified for infant patients, further studies, included high dose novel agents, target agents, were needed to improve outcomes for children and adult patients.
Supplementary Information
Acknowledgements
We thank the patients and their families for their approval; we also thank Kindstar Globalgene Technology, Inc for NGS sequencing.
Authors’ contributions
Lin Song and Jianwen Xiao analyzed and interpreted the patient data. Jinquan Wen, Min Zhou, Yali Shen, Yuting long, Yuxia Guo and Jianwen Xiao treat these patients and performed data, and Jianwen Xiao was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
The study was supported by the National Natural Science Foundation of China (Project No. 81900162) and the Chongqing Science and Technology Commission of China PR (Project No. cstc2018jsyj-jsyjX0015).
Availability of data and materials
The datasets used and/or analysed during the current study were available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study has been performed in accordance with the Declaration of Helsinki and it have been approved by Institutional Ethics Committee of Children Hospital, Chongqing Medical University (file number: 2015–28 and 2018–75); the study was also registered at the Chinese Clinical Trial Registry (registration numbers and date: ChiCTR-IPR-14005706, 04/06/2014; ChiCTR1900025690, 05/09/2019). An informed consent form was signed by the guardians of these patients and/or the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Min Zhou and Yali Shen contributed equally to this work.
References
- 1.El Chaer F, Keng M, Ballen KK. MLL-Rearranged Acute Lymphoblastic Leukemia. Curr Hematol Malig Rep. 2020;15(2):83–89. doi: 10.1007/s11899-020-00582-5. [DOI] [PubMed] [Google Scholar]
- 2.Meyer C, Burmeister T, Gröger D, Tsaur G, Fechina L, Renneville A, et al. The MLL recombinome of acute leukemias in 2017. Leukemia. 2018;32(2):273–284. doi: 10.1038/leu.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica. 2020;105(11):2524–2539. doi: 10.3324/haematol.2020.247031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agraz-Doblas A, Bueno C, Bashford-Rogers R, Roy A, Schneider P, Bardini M, Ballerini P, Cazzaniga G, Moreno T, Revilla C, Gut M, Valsecchi MG, Roberts I, Pieters R, De Lorenzo P, Varela I, Menendez P, Stam RW. Unraveling the cellular origin and clinical prognostic markers of infant B-cell acute lymphoblastic leukemia using genome-wide analysis. Haematologica. 2019;104(6):1176–1188. doi: 10.3324/haematol.2018.206375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato M, Manabe A. Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatr Int. 2018;60(1):4–12. doi: 10.1111/ped.13457. [DOI] [PubMed] [Google Scholar]
- 6.Gao C, Liu SG, Yue ZX, Liu Y, Liang J, Li J, Zhang YY, Yu JL, Wu Y, Lin W, Zheng HY, Zhang RD. Clinical-biological characteristics and treatment outcomes of pediatric pro-B ALL patients enrolled in BCH-2003 and CCLG-2008 protocol: a study of 121 Chinese children. Cancer Cell Int. 2019;14(19):293. doi: 10.1186/s12935-019-1013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai L, Cheng YF, Lu AD, Suo P, Wang Y, Zuo YX, et al. Prognosis of haploidentical hematopoietic stem cell transplantation in non-infant children with t(v;11q23)/MLL-rearranged B-cell acute lymphoblastic leukemia. Leuk Res. 2020;91:106333. doi: 10.1016/j.leukres.2020.106333. [DOI] [PubMed] [Google Scholar]
- 8.Pieters R, De Lorenzo P, Ancliffe P, Aversa LA, Brethon B, Biondi A, et al. Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the interfant-06 protocol: results from an international phase iii randomized study. J Clin Oncol. 2019;37(25):2246–2256. doi: 10.1200/JCO.19.00261. [DOI] [PubMed] [Google Scholar]
- 9.Cui Y, Zhou M, Zou P, Liao X, Xiao J. Mature B cell acute lymphoblastic leukaemia with KMT2A-MLLT3 transcripts in children: three case reports and literature reviews. Orphanet J Rare Dis. 2021;16(1):331. doi: 10.1186/s13023-021-01972-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou P, Zhou M, Wen J, Liao X, Shen Y, Liu H, et al. The long-term outcome and risk factors for precursor B cell acute lymphoblastic leukemia without specific fusion genes in Chinese children: experiences from multiple centers. Bosn J Basic Med Sci. 2021 doi: 10.17305/bjbms.2021.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M, Wen J, Guo Y, Shen Y, An X, Hu Y, et al. Clinical characterization and prognosis of T cell acute lymphoblastic leukemia with high CRLF2 gene expression in children. PLoS ONE. 2019;14(12):e0224652. doi: 10.1371/journal.pone.0224652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao X, Guo Y, Shen Y, Xiao J. Recurrent gastrointestinal hemorrhage in children with Philadelphia-Positive B-cell acute lymphoblastic leukemia treated with dasatinib: case reports. Case Rep Hematol. 2020;10(2020):5678210. doi: 10.1155/2020/5678210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harman JR, Thorne R, Jamilly M, Tapia M, Crump NT, Rice S, et al. A KMT2A-AFF1 gene regulatory network highlights the role of core transcription factors and reveals the regulatory logic of key downstream target genes. Genome Res. 2021;31(7):1159–1173. doi: 10.1101/gr.268490.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright RL, Vaughan AT. A systematic description of MLL fusion gene formation. Crit Rev Oncol Hematol. 2014;91(3):283–291. doi: 10.1016/j.critrevonc.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Yu W, Alikarami F, Qiu Q, Chen CH, Flournoy J, et al. Single-cell multiomics reveals increased plasticity, resistant populations, and stem-cell-like blasts in KMT2A-rearranged leukemia. Blood. 2022;139(14):2198–211. [DOI] [PMC free article] [PubMed]
- 16.Li JF, Dai YT, Lilljebjörn H, Shen SH, Cui BW, Bai L, et al. Transcriptional landscape of B cell precursor acute lymphoblastic leukemia based on an international study of 1,223 cases. Proc Natl Acad Sci U S A. 2018;115(50):E11711–E11720. doi: 10.1073/pnas.1814397115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stutterheim J, van der Sluis IM, de Lorenzo P, Alten J, Ancliffe P, Attarbaschi A, et al. Clinical implications of minimal residual disease detection in infants with KMT2A-rearranged acute lymphoblastic leukemia treated on the interfant-06 protocol. J Clin Oncol. 2021;39(6):652–662. doi: 10.1200/JCO.20.02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forgione MO, Mcclure BJ, Eadie LN, David TY, Deborah LW. KMT2A rearranged acute lymphoblastic leukaemia: Unravelling the genomic complexity and heterogeneity of this high-risk disease. Cancer Letters. 2020;469:410–18. doi: 10.1016/j.canlet.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Chaer FE, Keng M, Ballen KK. MLL-Rearranged Acute Lymphoblastic Leukemia[J] Curr Hematol Malig Rep. 2020;15(1):83–89. doi: 10.1007/s11899-020-00582-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study were available from the corresponding author on reasonable request.