FIGURE 1.
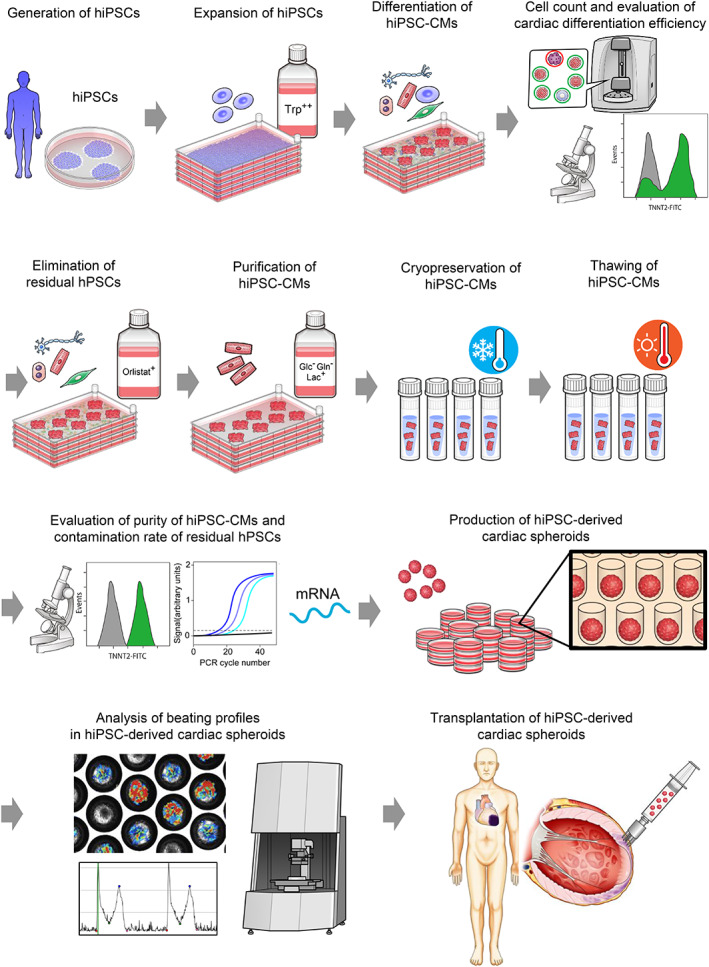
Scalable manufacturing of clinical‐grade hiPSC‐CMs. Tryptophan‐fortified media promotes the proliferation of hiPSCs. Large numbers of hiPSC‐CMs are induced in a multilayer culture plate. Cardiac differentiation efficiency is evaluated by cell count, flow cytometry, and immunostaining. Orlistat treatment selectively eliminates residual undifferentiated hPSCs. Then, hiPSC‐CMs were metabolically selected with glucose‐ and glutamine‐depleted lactate‐supplemented media. After purification, hiPSC‐CMs are isolated, harvested, and cryopreserved. The purity of hiPSC‐CMs and the contamination rate of residual undifferentiated hPSCs are assessed. After thawing, cardiac spheroids are produced. Beating profiles of cardiac spheroids are evaluated before transplantation. Cardiac spheroids are transplanted using our developed spheroids transplantation device.
