Visual Abstract
Key Words: atherosclerosis, coronary artery disease, endurance exercise, extreme sport, vascular stiffening
Abbreviations and Acronyms: CACS, coronary artery calcium score; CAD, coronary artery disease; CV, cardiovascular; MMP9, matrix metalloproteinase 9; NO, nitric oxide; Phe, phenylephrine; VSMC, vascular smooth muscle cell
Highlights
-
•
Long-term strenuous endurance training promotes a deleterious vascular remodeling, in contrast to the beneficial effects of moderate exercise.
-
•
Tunica media fibrosis, possibly mediated by miR-212, miR-132, and miR-146b down-regulation, and intrinsic vascular smooth muscle cell stiffening may contribute to aortic stiffening.
-
•
Endothelial function improves in a similar intensity after moderate and strenuous training. However, in the INT group, a larger NO-mediated vasorelaxation is compensated by more intense vasoconstriction, leading to a potentially unstable balance.
-
•
Strenuous exercise-induced vascular stiffening and changes in endothelial function remain after ceasing physical activity.
Summary
Moderate exercise has well-founded benefits in cardiovascular health. However, increasing, yet controversial, evidence suggests that extremely trained athletes may not be protected from cardiovascular events as much as moderately trained individuals. In our rodent model, intensive but not moderate training promoted aorta and carotid stiffening and elastic lamina ruptures, tunica media thickening of intramyocardial arteries, and an imbalance between vasoconstrictor and relaxation agents. An up-regulation of angiotensin-converter enzyme, miR-212, miR-132, and miR-146b might account for this deleterious remodeling. Most changes remained after a 4-week detraining. In conclusion, our results suggest that intensive training blunts the benefits of moderate exercise.
Regular physical activity is an efficient therapeutic strategy to reduce cardiovascular (CV) disease burden. Obesity, hypertension, and hyperlipidemia are among risk factors that are blunted after the initiation of physical activity programs, generally followed by a reduction in CV and all-cause mortality. Consequently, scientific societies and national public health policies promote moderate to intense regular physical activity in healthy individuals and most patients with heart disease.
With the advent and popularization of long- and ultralong-distance races, exercise is now performed at a much higher intensity and for longer periods than previously studied. In the last 2 decades, clinical and experimental data have revealed unexpected CV side effects of very high-intensity endurance training, particularly atrial fibrillation and ventricular arrhythmias in healthy and genetically predisposed athletes.1 More recent findings indicate that extreme exercise promotes adverse vascular remodeling. Small studies have observed that extreme long-distance runners have a higher than expected coronary artery calcium score,2 which translates into a high risk of CV complications.3 A large study has recently confirmed an increased coronary calcium burden in the most active individuals.4 These conclusions are not supported by contemporaneous clinical reports,5 and contrast with previous notions emphasizing the lack of a limit to exercise benefits.
As the potentially detrimental vascular consequences of physical activity remain controversial, the present study aimed to analyze the consequences of long-term, very high-intensity exercise on vascular structure and function, compared with those of moderate training, in an animal model, and to uncover the associated mechanisms.
Methods
Male Wistar rats (100-150 g; Charles River Laboratories) were randomly assigned to a sedentary group (SED) (n = 27) or to moderate training load (MOD) (35 cm/s, 45 minutes; n = 28) or intensive training load (INT) (60 cm/s, 60 minutes; n = 23) on a treadmill, 5 d/wk for 16 weeks.6 On the basis of previous reports,7 we estimate that training intensity roughly corresponded to 60% (MOD) and 85% (INT) of VO2max. An additional group of rats was kept sedentary for an additional 4-week period to test for the effects of detraining (DET) (DET groups: n = 8 for SED-DET, n = 8 for MOD-DET, n = 9 for INT-DET). A flowchart summarizing all animals that were used for this project is provided in Supplemental Figure 1. Animal care and experimentation conformed to the European Union (Directive 2010/63/EU) and Spanish (RD 53/2013) guidelines for the use of experimental animals. Approval was obtained from the local animal research ethics committee.
A comprehensive description of the research design and methodology is provided in the Supplemental Methods, Supplemental Figures 2 to 4 and Supplemental Table 1. Specifically, details on in vivo (the training protocol and groups, echocardiography, hemodynamic studies, and derived aortic stiffness estimation), ex vivo (vascular smooth muscle cellular stiffness assessment and vascular reactivity), histological and in vitro (microRNA profiling, PCR, and Western blotting) experiments are reported.
All experiments were analyzed by investigators blind to group assignment. The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Statistical analyses
Continuous variables are shown as mean ± SEM or median with 25th, 75th percentiles (Q1, Q3), depending on whether the normal distribution assumption was met (Q-Q plot of the residuals on parametric tests). Most comparisons were conducted with 1-way analysis of variance. Rat weight over the experimental period, histological analyses of intramyocardial arteries, and atomic force microscopy (AFM) measurements of vascular smooth muscle cell (VSMC) stiffness were modeled using a linear mixed-effects model (lme4 package in R, R Foundation for Statistical Computing). For weight change over time, group (SED, MOD, INT) and week (week 1-16) were used as fixed factors, and rat was entered as a random intercept. For the histological analyses of intramyocardial arteries, group (SED, MOD, INT) and ventricle (left ventricle, right ventricle) were used as fixed factors, and intramyocardial artery and rat as random factors; each intramyocardial artery was nested into ventricle, and ventricle was nested into rat. For VSMC stiffness assessment, group (SED, MOD, INT) was entered as a fixed factor in the model, and each measurement, cell, and rat as random factors; each measurement was nested into cell and nested into rat. In all cases, a significant P value for the overall analysis was followed by post hoc pairwise comparisons with least significant differences adjustment. Correlation between 2 continuous variables was tested by mean of linear regression, and R2 is shown as a measure of the explained variation.
Data for vascular reactivity were fitted in a nonlinear regression model with the following formula:
For each group, the drug concentration eliciting the 50% of the maximal response (EC50), and the maximal effect (Emax) were calculated and compared across groups.
Categorical variables are shown as n (%). Comparisons were carried out with Fisher exact test.
Statistical analyses were carried out with GraphPad Prism version 6.0 (GraphPad Software), Stata version 13.0 (StataCorp LLC), and R version 4.1.2. A P value <0.05 was considered significant.
Results
Weight gain over the 16-week training protocol was similar in both MOD and INT groups; SED rats gained significantly more weight (Supplemental Figure 5).
Exercise promotes load-dependent vascular structural remodeling
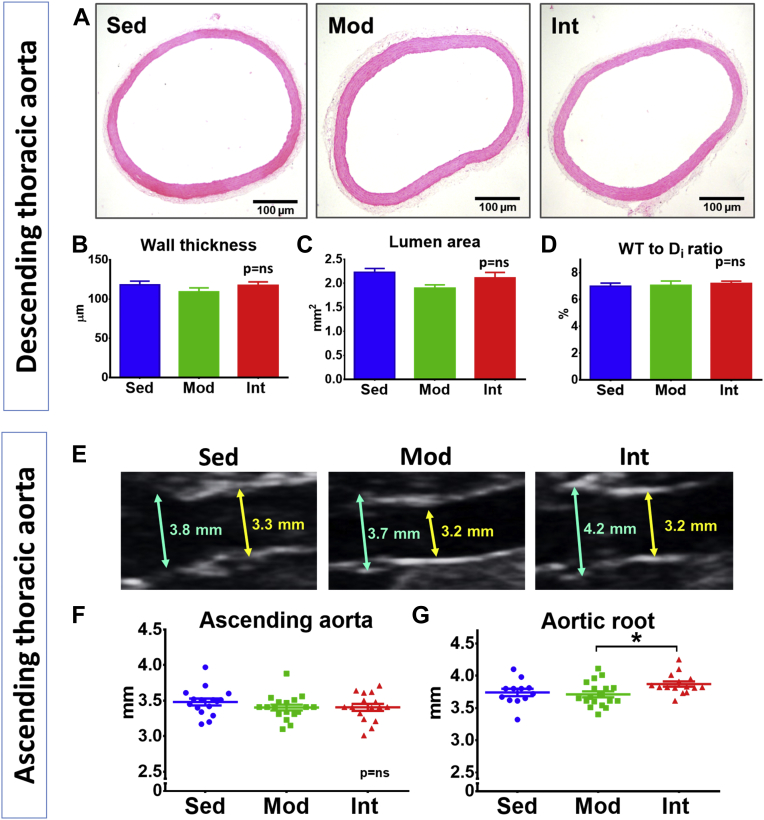
Vascular structural remodeling was assessed in elastic and muscular arteries. No significant changes were observed in wall thickness, lumen area, or wall thickness-to-lumen diameter ratio in histological preparations of either descending thoracic aorta (Figures 1A to 1D) or left carotid (Supplemental Figures 6A to 6D). Similarly, the echocardiographic assessment of the diameter of the ascending aorta showed no differences between groups (Figures 1E and 1F). In contrast, when the aorta was evaluated at a proximal level, we found that INT rats had a significantly larger aortic root than MOD and SED animals (Figure 1G).
Figure 1.
Aortic Morphological Changes
(A) Representative microphotographs of hematoxylin/eosin-stained thoracic aorta sections from sedentary (SED) (n = 10), moderate (MOD) (n = 6), and intensive (INT) (n = 6) training groups. (B to D) Quantification (mean ± SEM) of tunica media thickness (B), lumen area (C), and wall thickness to lumen diameter ratio (D). (E) Representative echocardiographic images of the root (blue arrow) and ascending thoracic aorta (yellow arrow). (F and G) Echocardiographic measurements (mean ± SEM) of ascending aorta (F) and aortic root (G) diameters (SED, n = 16; MOD, n = 17; INT, n = 16). All analyses were performed with a 1-way analysis of variance; the omnibus test was only significant for G at the P < 0.05 level. ∗P < 0.05.
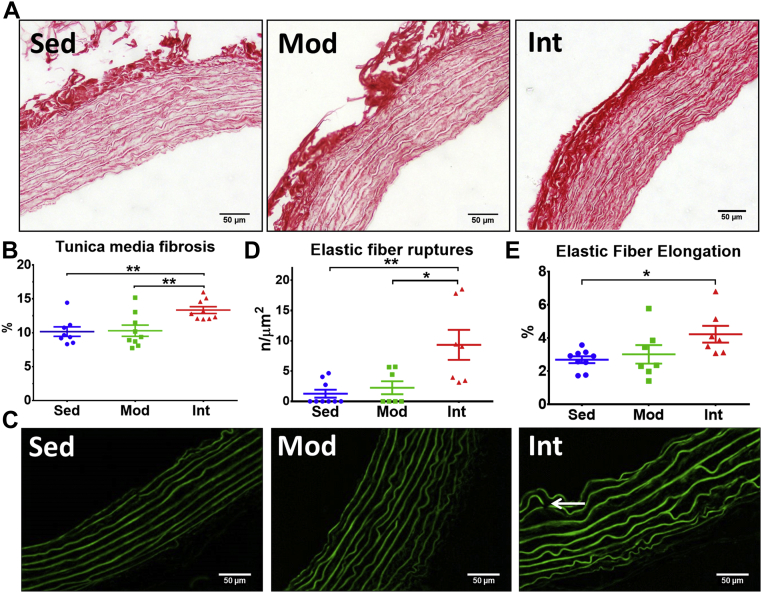
Fibrosis in the tunica media was assessed by means of Picrosirius-red staining (representative microphotographs in Figure 2A and Supplemental Figure 6A). Blind quantification found an increased collagen deposit in the INT group compared with the SED and MOD groups (Figure 2B for thoracic aorta and Supplemental Figure 6E for left carotid). Figure 2C shows representative images of the elastic laminae in the thoracic aorta as observed by autofluorescence. Elastic laminae had more discontinuities (Figure 2D) and were more elongated (Figure 2E) in INT rats compared with the MOD and SED groups. The number of laminae was similar in all 3 groups (Supplemental Figure 7).
Figure 2.
Aortic Tunica Media Structural Remodeling
(A) Representative Picrosirius-stained pictures of thoracic aorta sections. (B) Quantification of collagen content (mean ± SEM) in the tunica media (SED, n = 7; MOD, n = 9; INT, n = 9). (C) Representative images of elastic laminae revealed by autofluorescence. (D) Density of elastic lamina ruptures (white arrow in right panel of C) (mean ± SEM). (E) Assessment of elastic lamina elongation (mean ± SEM) (SED, n = 9; MOD, n = 7; INT, n = 7). All analyses were performed with a 1-way analysis of variance; omnibus tests were significant at the P < 0.01 (B and D) and the P < 0.05 (E) levels. ∗P < 0.05, ∗∗P < 0.01. Abbreviations as in Figure 1.
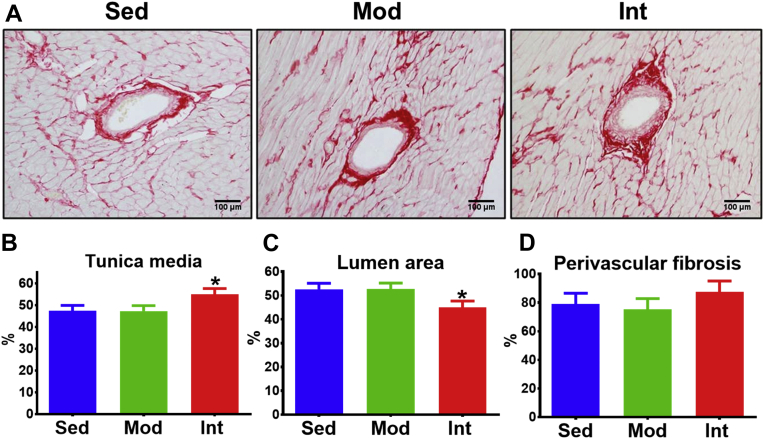
Midventricular intramyocardial arteries were selected to assess muscular arteries (Figure 3A). INT exercise was associated with a significant increase in the relative size of the tunica media area (Figure 3B) and, consequently, a narrower lumen (Figure 3C), compared with MOD and SED groups. No significant changes in perivascular fibrosis were noted (Figure 3D).
Figure 3.
Intramyocardial Vessels Remodeling
(A) Representative Picrosirius-stained pictures of intramyocardial arteries. (B to D) Area (mean ± SEM) of tunica media (B), lumen (C), and perivascular fibrosis (D) adjusted for total vessel area (SED, n = 9; MOD, n = 9; INT, n = 9). Analyses were performed with a linear mixed-effects model (vessel nested into ventricle, nested into rat). Omnibus tests were significant at the P < 0.05 level (B and C). ∗P < 0.05. Abbreviations as in Figure 1.
Vascular structural changes associated with mechanical properties
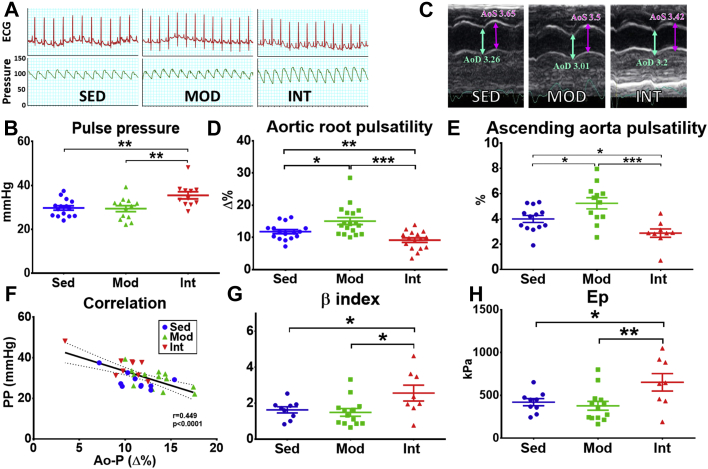
Based on an in vivo hemodynamic study and echocardiography, we tested whether increased fibrosis led to changes in mechanical properties of the vessel. Representative blood pressure recordings are shown in Figure 4A. Systolic, diastolic, and mean blood pressure were comparable between groups (Supplemental Figures 8A to 8C); however, pulse pressure was significantly higher in INT rats compared with MOD and SED (Figure 4B). Echocardiographic data (representative images in Figure 4C) demonstrated improved aortic root and ascending aorta pulsatility in MOD rats compared with SED rats, but INT exercise reverted this effect (Figures 4D to 4E): aortic pulsatility was lower in this group than in the other two. Pulse pressure was inversely correlated with aortic pulsatility (Figure 4F), suggesting that increased pulse pressure reflects an underlying low aortic distensibility. Finally, stiffness estimators (β-index and Ep) demonstrated a stiffer aorta in INT rats in comparison with MOD and SED rats (Figures 4G to 4H). Moreover, when both descending aorta fibrosis and pulsatility assessment were available, a negative but nonsignificant correlation was observed in these animals (Supplemental Figure 9).
Figure 4.
Aortic Stiffness Assessment
(A) Representative invasive blood pressure recordings in all groups. Both the electrocardiogram (top) and the central arterial pressure (bottom) are shown. (B) Quantification (mean ± SEM) of pulse pressure (PP), obtained from central pressure recordings in all groups (SED, n = 15; MOD, n = 13; INT, n = 11). (C) Representative echocardiographic images (M-mode) of ascending aorta in all groups. The maximum aortic systolic (AoS) (purple arrow) and aortic diastolic (AoD) (cyan arrow) diameters are shown. (D and E) Quantification (mean ± SEM) of aortic root (D) and ascending aorta (E) pulsatility obtained from echocardiographic data (SED, n = 16; MOD, n = 19; INT, n = 17). (F) Correlation between hemodynamic and echocardiographic data (Ao-P). (G and H) Estimation (mean ± SEM) of arterial stiffness by the β-index (G) and elastic modulus (Ep) (H) (SED, n = 9; MOD, n = 13; INT, n = 8). Most analyses (B, D, E, G, H) were performed with a 1-way analysis of variance; omnibus tests were significant at the P < 0.001 (D and E); P < 0.01 (B) and P < 0.05 (G and H) levels. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations as in Figure 1.
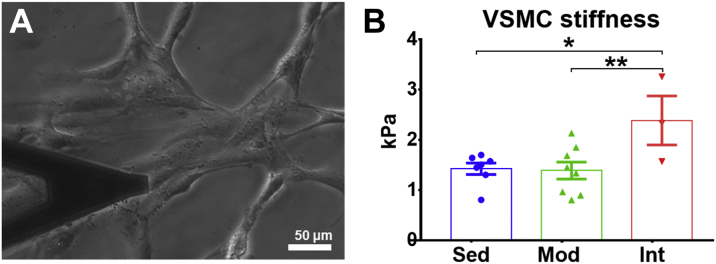
Intense training load promotes VSMC stiffening
To deepen into the mechanisms underlying aortic stiffening induced by INT training, we measured cellular stiffness of aortic VSMC with AFM. Figure 5 shows cultured VSMCs probed with AFM (Figure 5A) and presents results for the 3 groups under study (Figure 5B). Aortic VSMCs obtained from SED and MOD rats showed indistinguishable cellular stiffness. In keeping with a profibrotic environment and stiffer aorta, VSMC from INT rats were stiffer than SED and MOD rats.
Figure 5.
VSMC Stiffness Measurement By Atomic Force Microscopy
(A) Cultured vascular smooth muscle cells (VSMCs) with the AFM cantilever (left). (B) VSMC stiffness measurements (mean ± SEM) in all groups (right). Analyses were performed with a linear mixed-effects model (each measurement nested into cell, and each cell nested into rat). The omnibus test was significant at the P < 0.05 level. ∗P < 0.05; ∗∗P < 0.01.
Different exercise loads associated with changes in vascular function
Vascular function was assessed ex vivo in vascular reactivity experiments. Dose response curves are summarized in Figure 6, and main curve parameters are reported in Supplemental Table 2. In descending thoracic aortic rings, both MOD and INT rats showed better endothelial function than SED rats, as demonstrated by a significant increase in maximal carbachol-induced relaxation; no differences were found between MOD and INT rats (Figure 6A). Endothelial-mediated vasorelaxation was almost completely blocked after incubating with NO-synthesis inhibitor L-NMMA, and differences between sedentary and exercise groups disappeared (Figure 6B).
Figure 6.
Vascular Reactivity of the Thoracic Aorta
(A and B) Dose-response vasorelaxation curves to Carbachol (CCh) in intact endothelium conditions (A) (SED, n = 19; MOD, n = 21; INT, n = 11) and nitric oxide (NO)-independent conditions after LNMMA incubation (B) (SED, n = 20; MOD, n = 19; INT, n = 15). (C and D) Dose-response vasoconstriction curves to Phenylephrine (Phe) in intact endothelium conditions (C) (SED, n = 9; MOD, n = 12; INT, n = 8) and NO-independent conditions after LNMMA incubation (D). Shaded areas represent 95% CI. Comparisons performed with sum-of-squares F tests by comparing fitted logEC50 and maximum effect in 3-parameter equations. ∗∗P < 0.01. Abbreviations as in Figure 1.
Improved endothelial function was further supported by a decreased maximal contractile response to phenylephrine (Phe) in endothelium-intact aortic rings from both MOD and INT rats compared with the SED group (Figure 6C). Surprisingly, when intrinsic NO production was blocked by preincubating aortic rings with L-NMMA, INT aortas showed a markedly increased contraction response to Phe (Figure 6D), suggesting a major contribution of NO to the decrease in Phe-induced contraction.
Monocyte infiltration
Monocyte infiltration was assessed in CD68-immunostained samples as an early atherosclerosis marker (Supplemental Figure 10A). Both MOD and INT training significantly reduced monocyte density in the tunica intima and media compared with SED rats (Supplemental Figure 10B).
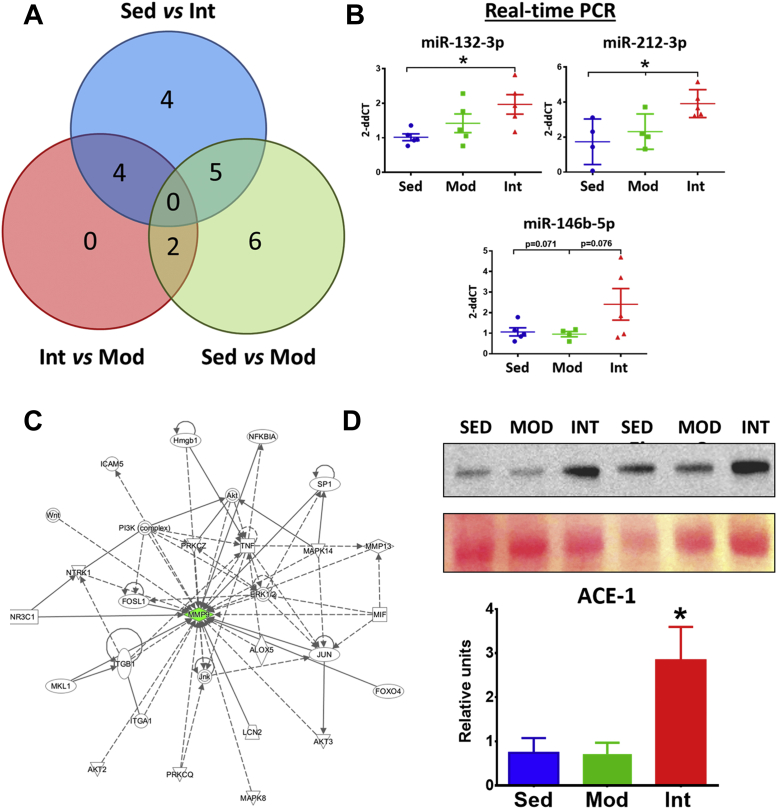
Differentially expressed miRNAs associated with exercise load
A microarray was used to investigate postexercise miRNA regulation of vascular remodeling. Overall, 21 miRNAs were found to be differentially expressed in at least 1 of the possible pairwise comparisons (Figure 7A).
Figure 7.
Mechanisms of Exercise-Induced Vascular Remodeling
(A) Venn diagram showing the 21 unique miRNAs that were deregulated between the different comparisons in the miRNA microarray analysis of thoracic aorta. (B) Relative expression (mean ± SEM) by RT-PCR of the miRNA that were deregulated in the INT group in comparison to both SED and MOD groups in the miRNA array. (C) Ingenuity analysis of the validated targets of the 3 selected miRNAs. Analyses point to MMP9 as a central component of the largest network including validated targets of miR-132-3p, miR-212-3p, and miR-146b-5p. (D) Representative ACE-1 blots and Ponceau-stained membranes (top), and relative quantification (mean ± SEM) of ACE-1 protein in all groups (bottom). (SED, n = 6; MOD, n = 7; INT, n = 6). Analyses were performed with 1-way analysis of variance; omnibus tests were significant at the P < 0.05 level. ∗P < 0.05.
To determine miRNAs involvement in the deleterious vascular effects of a high training load, we focused on those selectively modified by INT exercise compared with MOD and SED groups. In total, 4 of the 21 miRNAs (miR-132-3p, miR-212-3p, miR-146b-5p, and miR-326-5p) were deregulated in INT rats (Supplemental Figure 11). Up-regulation of 3 of these microRNAs (miR-132-3p, miR-212-3p, and miR-146b-5p) was confirmed with RT-PCR (Figure 7B), while the expression of miR-326-5p was exceedingly low.
We explored the potential involvement of these 3 miRNAs in the pathogenic mechanisms behind tunica media fibrosis. Validated targets of miR-132-3p, miR-212-3p, and miR-146b-5p were used to build molecular networks by means of Ingenuity analysis. Three networks eventually emerged from this analysis (Supplemental Table 3); the largest one, in which matrix metalloproteinase 9 (MMP9) was a central component, is depicted in Figure 7C.
Angiotensin-converter enzyme synthesis
Previous work demonstrated that angiotensin II induces miR-212, miR-132, and miR-146b up-regulation.8,9 We quantified the levels of angiotensin-converting enzyme (ACE)-1, the main enzyme promoting angiotensin-I to -II conversion, in aortic tissue, finding significantly higher ACE-1 protein levels in the INT group compared with the MOD and SED groups (Figure 7D).
Remodeling after detraining
After a 4-week detraining, weight was similar among groups (Supplemental Table 4). Vascular reactivity and morphometric results are displayed in Supplemental Figure 12. Four weeks after the last training session, an improved endothelial function was still evident in MOD- and INT-DET rats compared with SED-DET rats (Supplemental Figure 12A). Nevertheless, INT-DET still showed an increased NO-independent aortic contraction (Supplemental Figure 12B). Morphometric parameters were unchanged after the 4-week detraining period (Supplemental Figures 12C to 12E), except for an increased tunica media fibrosis in INT-DET rats (Supplemental Figure 12F).
Discussion
The present study showed that a long-term intensive exercise load promotes adverse changes in the structural and functional properties of elastic and muscular arteries in an animal model. The 4 main findings were: 1) intensive exercise promoted tunica media fibrosis and stiffening, elastic laminae abnormalities, thickening of intramyocardial arteries, and VSMC stiffening; 2) endothelial function was similarly improved after moderate and intensive training, although the balance between vasodilator and vasoconstrictor mediators was disturbed in the latter; 3) exercise-induced remodeling persisted after a short-term detraining: and 4) microRNA remodeling and the renin-angiotensin system may mediate the heightened fibrosis and vascular stiffening occurring in intensively-trained animals.
Overall, tunica media fibrosis and stiffening, narrowing of intramyocardial arteries, and an imbalance in vasodilator-vasoconstrictor mediators qualitatively characterized the transition from the benefits of moderate exercise to the potentially deleterious consequences of intensive exercise.
Very high-intensity exercise may increase coronary artery disease burden and compromise CV outcomes
Although the benefits of moderate physical activity have been acknowledged for years, potentially deleterious vascular consequences of very high-intensity exercise have only recently been claimed. The most compelling evidence came from a large British retrospective study involving more than 1 million women.3 In that study, limited (≤4 d/wk) strenuous physical activity decreased the risk of myocardial infarction and cerebrovascular disease, compared with those not exercising at all. In contrast, when strenuous physical activity was performed >4 d/wk, the cerebrovascular risk was increased, and cardiac risk rose when strenuous exercise was performed daily.
A higher-than-expected coronary artery disease (CAD) burden has been observed in some athletes undergoing very high-intensity training, potentially accounting for the increased CV risk. In a seminal work,2 coronary calcium scores were higher in marathon runners compared with a risk factor–matched cohort. Some,4,10, 11, 12 but not all subsequent works5,13 confirmed the association between marathon running and an increased coronary calcium score. Data from the Sharma group support that increased coronary calcification in athletes is independent of the presence of classic risk factors.12 Relatively short high-intensity training periods seem to be safe and beneficial,14 but maladaptive remodeling may occur after very long-term training.11,12 In this regard, our animal model has been estimated to be roughly equivalent to a 10- to 12-year regular strenuous training period in humans.15,16
Intriguingly, coronary calcification has been suggested to contribute to plaque stability in athletes.11,12 However, calcium score has been shown to retain its predictive power in marathon runners.17 The Cooper Clinic Longitudinal Study suggested that amongst the most active individuals (ie, those performing >3,000 MET-min/wk), a coronary calcium score (CACS) >100 was associated with a 9-fold higher risk of CV death compared with those with a low CACS.4 Moreover, DeFina et al4 also found that mortality was decreased by 45% (95% CI: 6%-68%) in the most active individuals among those without coronary calcification (CACS <100), but not in those with a high coronary calcium burden (HR: 0.77 [95% CI: 0.52-1.15]). Thereby, although biologically plausible, evidence is still needed to support that coronary calcification translates into lower CV risk in athletes.18
Early atherosclerosis features are absent in very intense exercise-induced deleterious vascular remodeling
Atherosclerosis underlies CAD in most patients with ischemic heart disease. Rodents are known to be atherosclerosis-resistant, but monocyte infiltration and endothelial dysfunction have been reported in some models.19 Notably, both preatherosclerotic processes apparently improved in moderate and heavily trained rats to a similar extent. However, our data point to an imbalance between vasodilator and vasoconstrictor mediators in the MOD and INT groups. The response to Phe was similar in the MOD and INT groups under baseline conditions, but a larger contractile response in NO-independent conditions in the INT group pointed to an increased NO synthesis compensated by increased Phe-induced contractile mediators. The newly established balance could be sensitive to NO synthesis disruption after the (future) development of additive risk factors.
Exercise-induced maladaptation targets the tunica media
In contrast to the relatively preserved endothelium, the tunica media was overtly damaged in INT rats. Mechanical properties of the arterial wall are determined by the composition of the extracellular matrix, particularly collagen content and elastic laminae integrity. In our animal model, an intensive exercise load induced tunica media fibrosis and elastic fiber ruptures, providing the basis for a stiffer aorta and a larger pulse pressure, a stiffness marker predicting CV risk.20 Moreover, stiffer VSMC in the aorta of heavily trained rats could also contribute to increased vascular stiffness, as previously demonstrated.21 This finding is consistent with some studies showing stiffer arteries in athletes compared with active counterparts,22 although conflicting data have also been published.23
Vascular remodeling after a high exercise load resembles the progressive process involving tunica media degeneration and aortic stiffening and dilation termed “vascular aging.”24 Vascular aging has been claimed to result from the fatigue and fracture of elastic laminae in the proximal aorta24,25 and underlies CV events in patients without atherosclerosis. In our model, repetitive large increases in cardiac output during intensive exercise bouts may have resulted in collagen deposition and elastin fragmentation, contributing to decreased arterial compliance.
Most clinical studies have found that increased CAD in athletes is driven by larger calcified coronary plaques,2,12 and that athletes and nonathletes have similar noncalcified plaques. In the absence of atherosclerosis markers, but with a prominent vascular tunica media damage, it is plausible that coronary calcification in athletes involves a process initiated in the tunica media. Further studies are needed to confirm this hypothesis.
A profibrotic environment underlies vascular remodeling after intensive exercise
Our findings are consistent with a profibrotic environment underlying a deleterious vascular remodeling after intensive training, potentially mediated by activation of the renin/angiotensin system and miR-132, miR-212, and miR-146b up-regulation.
The miR-132/212 family includes highly conserved miRNAs that, when up-regulated, contribute to cardiac hypertrophy, hypertension, and vascular remodeling.26 Ablation of the miR-212/132 cluster reduces fibrosis in the kidney.27 miR-146a/b are up-regulated in human atherosclerotic plaques,28 potentially playing a role in VSMC proliferation.29 The participation of these 3 miRNAs in strenuous exercise-induced deleterious vascular remodeling is supported by clinical observations. In amateur athletes, circulating miR-132 increases after running a marathon but not after a shorter 10-km race.30 Similarly, miR-146 family member miR-146a is increased in plasma after a prolonged aerobic exercise.31
In our study, Ingenuity pathway analysis suggested that MMP9 down-regulation could play an important role in the vascular maladaptive process. MMP9 is a zinc-metalloproteinase enzyme that degrades extracellular matrix proteins, such as collagen I, II, III, IV, and V and fibronectin, but also regulates a complex network of inflammatory and fibrosis-related cytokines.32 Circulating MMP9 levels are generally increased in untreated hypertensive patients,33 but it is unclear whether MMP9 triggers vascular remodeling or works as a compensatory mechanism in response to increased collagen synthesis. In contrast, primary MMP9 down-regulation appears to mediate a reduction in collagen degradation and a subsequent increase in vascular stiffness; MMP9 knockout mice have increased vessel stiffness and pulse pressure at the onset of Ang II-induced hypertension.34
miR-212, miR-132, and miR-146b are tightly regulated by angiotensin-II (Ang-II).8,9 ACE-1, a central enzyme in Ang-II synthesis obtained by cleaving Ang-I into Ang-II, was found to be up-regulated in the aorta of INT-trained rats, suggesting that extreme exercise may promote adverse vascular remodeling through local angiotensin activation and subsequent miRNA deregulation. Consistent with this notion, treatment with losartan, an AT1 receptor blocker, prevented exercise-induced myocardial fibrosis in rats.35 Confirmation of these findings warrants further studies.
Clinical implications: Outcomes affected by physical activity have a U-shaped relationship
In this paper we show that vascular remodeling depends on the load of physical activity in a nonlinear manner, which could be the basis of the deleterious vascular consequences of very high-intensity exercise that was observed in some clinical studies. Whether such increased CV risk, along with increased AF risk,1 has an impact on overall mortality remains controversial. In the general population, moderate exercise is associated with reduced mortality, but mortality does not further decrease with very high doses of physical activity.4 Although top-level athletes live longer than nonathletes,36 this survival benefit may rely on physical activity but also on related dietary and socioeconomical factors. Altogether, these data suggest that the largest benefits of exercise are obtained when practiced at a moderate load.
Study limitations
First, translation from animal models to human remains challenging, particularly regarding the effect of different training loads. Clinical relevance of our work is supported by confirmation in athletes of previous results in the same animal model. For example, exercise-induced atrial fibrosis was initially proposed in this model16 and recently confirmed in athletes.37 Of note, we only used young male rats, and thus cannot generalize our findings to remodeling in female rats or later stages of life. Sex differences may be particularly relevant. Most reports showing an increased coronary calcification have been conducted in male athletes,4,11,12 but worse outcomes have been particularly proven in the most active women.3 Whether the consequences of long-term strenuous exercise, both from vascular remodeling and clinical points of view, are similar in men and women needs to be tested. Our model recapitulates long-term exercise,15 and shorter training periods might yield different conclusions.14 Moreover, only endurance training was tested, and conclusions cannot be extrapolated to other sorts of sports. Second, stress should be considered a potential confounding factor. Maximum care was taken to minimize electrical shocks, and rats that did not properly adapt to the treadmill training were excluded. Previous reports noted no evident stress in intensively trained rats.16
Conclusions
In a chronic animal model, intense exercise promoted tunica media fibrosis and stiffening of the aorta, along with narrowing of the intramyocardial arteries, potentially through a process mediated by the renin-angiotensin-aldosterone system, miR-212/132 and miR-146b, and MMP9. Moderate and intense training loads similarly improved vascular function, although the vasodilation-vasoconstriction balance was disturbed by intensive training. Overall, the largest benefits of physical activity were obtained when practiced at a moderate load.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Although moderate-endurance exercise helps prevent CV disease, concerns have been raised about the consequences of strenuous physical activity. In this study, we show in an animal model that very long-term, high-intensity physical activity promotes undesirable consequences in arterial bed structure, mechanical properties, and function, including aortic stiffening, tunica media fibrosis, and an imbalance in vascular reactivity mediators.
TRANSLATIONAL OUTLOOK: Our work suggests that long-term, high-intensity endurance exercise promotes vascular stiffening by damaging the tunica media. The effects of types of sport other than endurance training need to be studied. Large studies in athletes engaged in very high-intensity physical activity are warranted to confirm our results and assess long-term outcomes. However, robust, well-controlled confirmatory studies in humans providing accurate exercise estimates are difficult to conduct because of the long but variable nature of exercise practice.
Funding Support and Author Disclosures
This work was partially supported by grants from the Instituto de Salud Carlos III (PI13/01580, PI16/00703, PI19/00443), co-funded by the European Union; CERCA program/Generalitat de Catalunya; CIBERCV (16/11/00354); and Spanish Ministry of Economy and Competitiveness (DPI2017-83721-P). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Dr Victor Peinado for his support and advice on vascular reactivity experiments, and Nadia Castillo for excellent technical assistance. The miRNA experiments and analyses were performed at the Genomics Core Facility and Bioinformatics platforms of IDIBAPS, Barcelona.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section as well as supplemental tables and figures, please see the
Contributor Information
Lluís Mont, Email: lmont@clinic.cat.
Eduard Guasch, Email: eguasch@clinic.cat.
Appendix
.References
- 1.Guasch E., Mont L. Diagnosis, pathophysiology, and management of exercise-induced arrhythmias. Nat Rev Cardiol. 2017;14:88–101. doi: 10.1038/nrcardio.2016.173. [DOI] [PubMed] [Google Scholar]
- 2.Möhlenkamp S., Lehmann N., Breuckmann F., et al. Running: the risk of coronary events: prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J. 2008;29:1903–1910. doi: 10.1093/eurheartj/ehn163. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong M.E.G., Green J., Reeves G.K., Beral V., Cairns B.J., Million Women Study Collaborators Frequent physical activity may not reduce vascular disease risk as much as moderate activity: large prospective study of women in the United Kingdom. Circulation. 2015;131:721–729. doi: 10.1161/CIRCULATIONAHA.114.010296. [DOI] [PubMed] [Google Scholar]
- 4.DeFina L.F., Radford N.B., Barlow C.E., et al. Association of all-cause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol. 2019;4:174–181. doi: 10.1001/jamacardio.2018.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts W.O., Schwartz R.S., Kraus S.M., et al. Long-term marathon running is associated with low coronary plaque formation in women. Med Sci Sports Exerc. 2017;49:641–645. doi: 10.1249/MSS.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 6.Sanz-de la Garza M., Rubies C., Batlle M., et al. Severity of structural and functional right ventricular remodeling depends on training load in an experimental model of endurance exercise. Am J Physiol Heart Circ Physiol. 2017;313:H459–H468. doi: 10.1152/ajpheart.00763.2016. [DOI] [PubMed] [Google Scholar]
- 7.Wisløff U., Helgerud J., Kemi O.J., Ellingsen O. Intensity-controlled treadmill running in rats: VO(2 max) and cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2001;280:H1301–H1310. doi: 10.1152/ajpheart.2001.280.3.H1301. [DOI] [PubMed] [Google Scholar]
- 8.Jiang X., Ning Q., Wang J. Angiotensin II induced differentially expressed microRNAs in adult rat cardiac fibroblasts. J Physiol Sci. 2013;63:31–38. doi: 10.1007/s12576-012-0230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eskildsen T.V., Jeppesen P.L., Schneider M., et al. Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int J Mol Sci. 2013;14:11190–11207. doi: 10.3390/ijms140611190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz R.S., Kraus S.M., Schwartz J.G., et al. Increased coronary artery plaque volume among male marathon runners. Mo Med. 2014;111:87–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Aengevaeren V.L., Mosterd A., Braber T.L., et al. Relationship between lifelong exercise volume and coronary atherosclerosis in athletes. Circulation. 2017;136:138–148. doi: 10.1161/CIRCULATIONAHA.117.027834. [DOI] [PubMed] [Google Scholar]
- 12.Merghani A., Maestrini V., Rosmini S., et al. Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation. 2017;136:126–137. doi: 10.1161/CIRCULATIONAHA.116.026964. [DOI] [PubMed] [Google Scholar]
- 13.Bertoni A.G., Whitt-Glover M.C., Chung H., et al. The association between physical activity and subclinical atherosclerosis: The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169:444–454. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhuva A.N., D’Silva A., Torlasco C., et al. Training for a first-time marathon reverses age-related aortic stiffening. J Am Coll Cardiol. 2020;75:60–71. doi: 10.1016/j.jacc.2019.10.045. [DOI] [PubMed] [Google Scholar]
- 15.Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med. 2013;4:624–630. [PMC free article] [PubMed] [Google Scholar]
- 16.Guasch E., Benito B., Qi X., et al. Atrial fibrillation promotion by endurance exercise. J Am Coll Cardiol. 2013;62:68–77. doi: 10.1016/j.jacc.2013.01.091. [DOI] [PubMed] [Google Scholar]
- 17.Möhlenkamp S., Leineweber K., Lehmann N., et al. Coronary atherosclerosis burden, but not transient troponin elevation, predicts long-term outcome in recreational marathon runners. Basic Res Cardiol. 2014;109:391. doi: 10.1007/s00395-013-0391-8. [DOI] [PubMed] [Google Scholar]
- 18.Aengevaeren V.L., Mosterd A., Sharma S., et al. Exercise and coronary atherosclerosis: observations, explanations, relevance, and clinical management. Circulation. 2020:1338–1350. doi: 10.1161/CIRCULATIONAHA.119.044467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campi P., Herrera B.S., De Jesus F.N., et al. Endothelial dysfunction in rats with ligature-induced periodontitis: Participation of nitric oxide and cycloxygenase-2-derived products. Arch Oral Biol. 2016;63:66–74. doi: 10.1016/j.archoralbio.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Millar J.A., Lever A.F. Implications of pulse pressure as a predictor of cardiac risk in patients with hypertension. Hypertension. 2000;36:907–911. doi: 10.1161/01.hyp.36.5.907. [DOI] [PubMed] [Google Scholar]
- 21.Zhou N., Lee J.-J., Stoll S., et al. Inhibition of SRF/myocardin reduces aortic stiffness by targeting vascular smooth muscle cell stiffening in hypertension. Cardiovasc Res. 2017;113:171–182. doi: 10.1093/cvr/cvw222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burr J.F., Drury C.T., Phillips A.a., Ivey A., Ku J., Warburton D.E.R. Long-term ultra-marathon running and arterial compliance. J Sci Med Sport. 2014;17:322–325. doi: 10.1016/j.jsams.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Pressler A., Suchy C., Friedrichs T., et al. Running multiple marathons is not a risk factor for premature subclinical vascular impairment. Eur J Prev Cardiol. 2017;24:1328–1335. doi: 10.1177/2047487317713326. [DOI] [PubMed] [Google Scholar]
- 24.O’Rourke M.F., Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 25.Tsamis A., Krawiec J.T., Vorp D.A. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J R Soc Interface. 2013;10(83):20121004. doi: 10.1098/rsif.2012.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumarswamy R., Volkmann I., Beermann J., et al. Vascular importance of the miR-212/132 cluster. Eur Heart J. 2014;35:3224–3231. doi: 10.1093/eurheartj/ehu344. [DOI] [PubMed] [Google Scholar]
- 27.Bijkerk R., de Bruin R.G., van Solingen C., et al. Silencing of microRNA-132 reduces renal fibrosis by selectively inhibiting myofibroblast proliferation. Kidney Int. 2016;89:1268–1280. doi: 10.1016/j.kint.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 28.Raitoharju E., Lyytikainen L.-P., Levula M., et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011;219:211–217. doi: 10.1016/j.atherosclerosis.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Sun S., Zheng B., Han M., et al. miR-146a and Krüppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep. 2011;12:56–62. doi: 10.1038/embor.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Gonzalo-Calvo D., Dávalos A., Montero A., et al. Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J Appl Physiol. 2015;119:124–134. doi: 10.1152/japplphysiol.00077.2015. [DOI] [PubMed] [Google Scholar]
- 31.Baggish A.L., Park J., Min P.-K., et al. Rapid upregulation and clearance of distinct circulating microRNAs after prolonged aerobic exercise. J Appl Physiol. 2014;116:522–531. doi: 10.1152/japplphysiol.01141.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou S., Wang Y., Tan Y., et al. Deletion of metallothionein exacerbates intermittent hypoxia-induced oxidative and inflammatory injury in aorta. Oxid Med Cell Longev. 2014;2014:141053. doi: 10.1155/2014/141053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed S.H., Clark L.L., Pennington W.R., et al. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 34.Flamant M., Placier S., Dubroca C., et al. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 2007;50:212–218. doi: 10.1161/HYPERTENSIONAHA.107.089631. [DOI] [PubMed] [Google Scholar]
- 35.Gay-Jordi G., Guash E., Benito B., et al. Losartan prevents heart fibrosis induced by long-term intensive exercise in an animal model. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marijon E., Tafflet M., Antero-Jacquemin J., et al. Mortality of French participants in the Tour de France (1947-2012) Eur Heart J. 2013;34:3145–3150. doi: 10.1093/eurheartj/eht347. [DOI] [PubMed] [Google Scholar]
- 37.Peritz D.C., Catino A.B., Csecs I., et al. High-intensity endurance training is associated with left atrial fibrosis. Am Heart J. 2020;226:206–213. doi: 10.1016/j.ahj.2020.05.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.