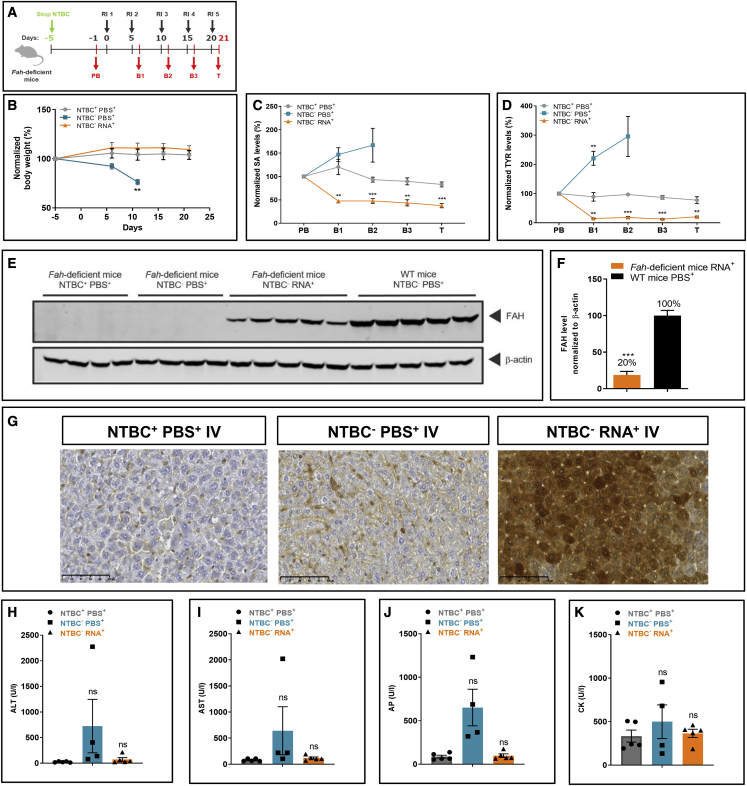
Figure 2.
Repeated FAH mRNA-LNPs injections i.v. rescue Fah-deficient mice from death
(A) Experiment schedule. NTBC supplementation was withdrawn on day 5 prior to injections. Blood was collected on day 1 before treatment (PB), at intermittent times 24 h after each injection (i.e. B1 day 6; B2 day 11; B3 day 16), and on termination day 21. NTBC+PBS+ received continuous NTBC supplementation and repeated PBS injections throughout the experiment. NTBC−PBS+ mice were stopped for NTBC supplementation on day 5 prior to treatment and injected with PBS repeatedly. NTBC−RNA+ mice were withdrawn from NTBC supplementation on day 5 prior to treatment; LNP-formulated therapeutic FAH mRNA was injected i.v. every 5 days (i.e., days 0, 5, 10, 15, and 20; repeated injection 1–5). (B) Normalized body weights of Fah-deficient mice. (C) Normalized serum SA and (D) TYR levels in Fah-deficient mice (five mice/group). (E) Western blot analyses of FAH protein in Fah-deficient and WT mouse livers, normalized to β-actin loading control (repeated injection; WT mice received single PBS injection). (F) Quantitation of FAH protein bands normalized to β-actin loading control. (G) Representative images of immunohistochemical FAH protein staining on paraffin-embedded Fah-deficient mouse liver sections after repeated i.v. injections of FAH mRNA-LNPs. (H) Alanine transaminase (ALT), (I) aspartate transaminase (AST), (J) alkaline phosphatase (AP), and (K) creatine kinase (CK) were evaluated in Fah-deficient mouse serum at termination. Data are the means ± SEM. Significantly different from the control group: ∗∗p < 0.01, ∗∗∗p < 0.001 (two-tailed Student’s t test). All NTBC−PBS+Fah-deficient mice were euthanized before the scheduled end of the experiment due to weight loss.