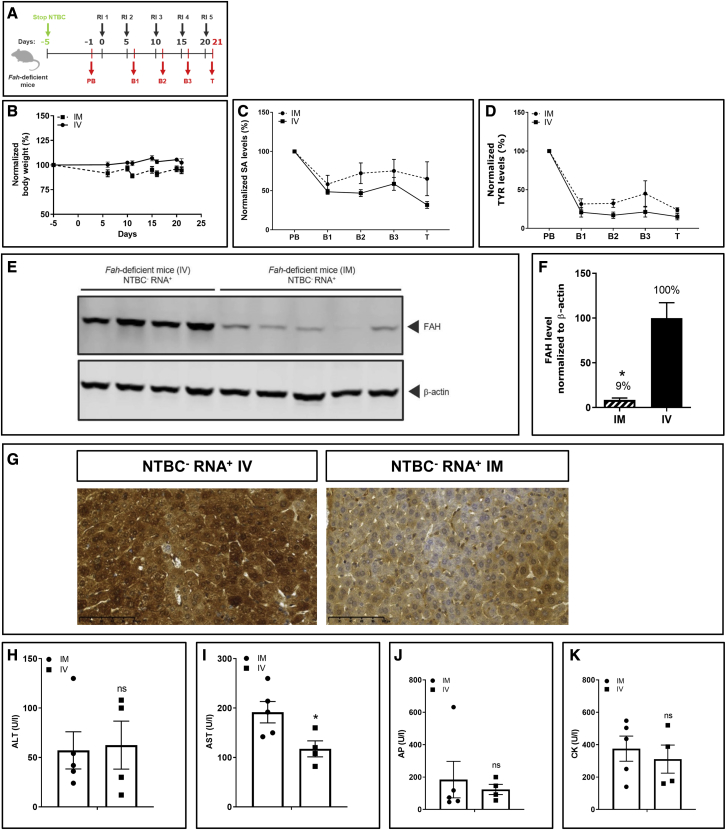
Figure 3.
Repeated i.m. and i.v. injections with FAH mRNA-LNPs in Fah-deficient mice exhibit comparable therapeutic effects
(A) NTBC supplementation was withdrawn from Fah-deficient mice. Blood was collected 1 day before treatment (PB; day −1), at intermediate bleeding time points 24 h after injections (B1–B3 on days 6, 11, and 16), and on termination day (T; day 21) (Fah-deficient mice injected i.m., n = 5; Fah-deficient mice injected i.v., n = 4). (B) Normalized body weights. (C) Normalized serum SA levels. (D) Normalized serum TYR levels. (E) Western blot analyses of FAH protein in livers of Fah-deficient mice dissected on day 21; β-actin served as loading control. (F) Quantitation of FAH protein bands normalized to β-actin. FAH amount in livers of Fah-deficient mice injected repeatedly i.v. was set to 100%. (G) Representative images of immunohistochemical FAH protein staining on paraffin-embedded Fah-deficient mouse liver sections after repeated i.v. versus i.m. injections of FAH mRNA-LNPs. (H) Alanine transaminase (ALT), (I) aspartate transaminase (AST), (J) AP, and (K) CK were evaluated in Fah-deficient mouse serum at termination. Data are the means ± SEM. Significantly different from the control group: ∗∗p < 0.01, ∗∗∗p < 0.001 (two-tailed Student’s t test).