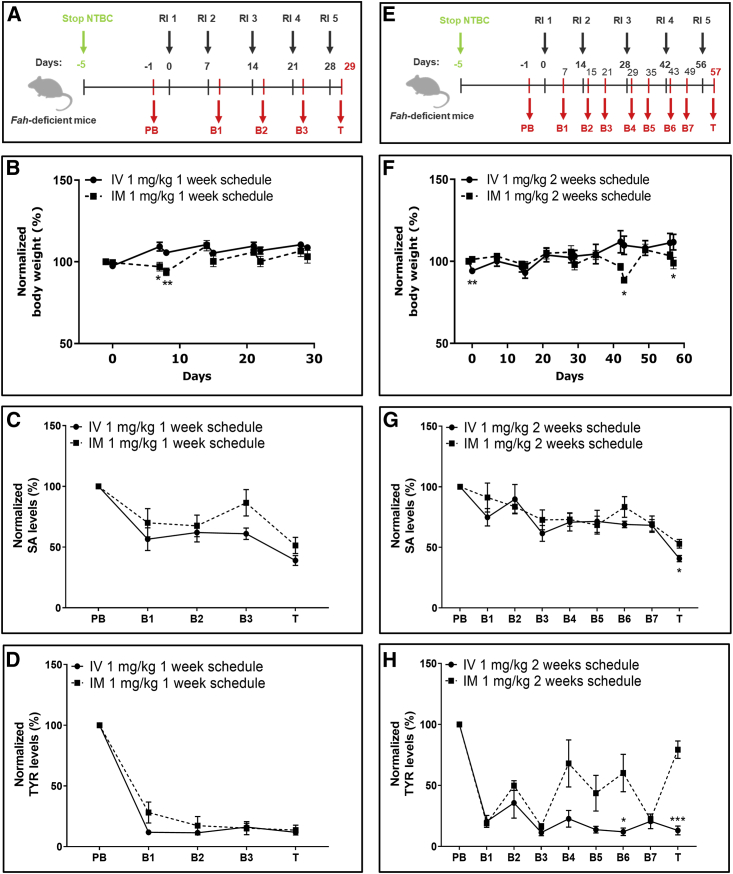
Figure 6.
Interval extension studies for repeated i.v. and i.m. injections of FAH mRNA-LNPs into Fah-deficient mice
(A) Weekly experimental protocol. NTBC supplementation was withdrawn from Fah-deficient mice for 5 days. Subsequently, five mice/group were repeatedly injected (1–5) i.v. or i.m. at 1-week intervals with 1 mg/kg FAH mRNA-LNPs; blood was collected (PB, B1–B3, and T) according to the schedule shown. (B) Normalized body weights recorded at 1-week intervals. (C) Normalized serum SA and (D) TYR levels recorded at 1-week interval. (E) Biweekly experimental protocol. NTBC supplementation was withdrawn from Fah-deficient mice for 5 days. Subsequently, five mice/group were repeatedly injected (1–5) i.v. or i.m. at 2-week intervals with 1 mg/kg FAH mRNA-LNPs; blood was collected (PB, B1–B7, and T) according to the schedule shown. (F) Normalized body weights determined at 2-week intervals. (G) Normalized serum SA and (H) TYR levels quantified at 2-week intervals. Data are the means ± SEM. Significantly different between i.v. and i.m. injected mice: ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (two-tailed Student’s t test).