Fig. 3 |. Preclinical in vitro model SCFM2 provides a platform to study biological implications of microbiogeography.
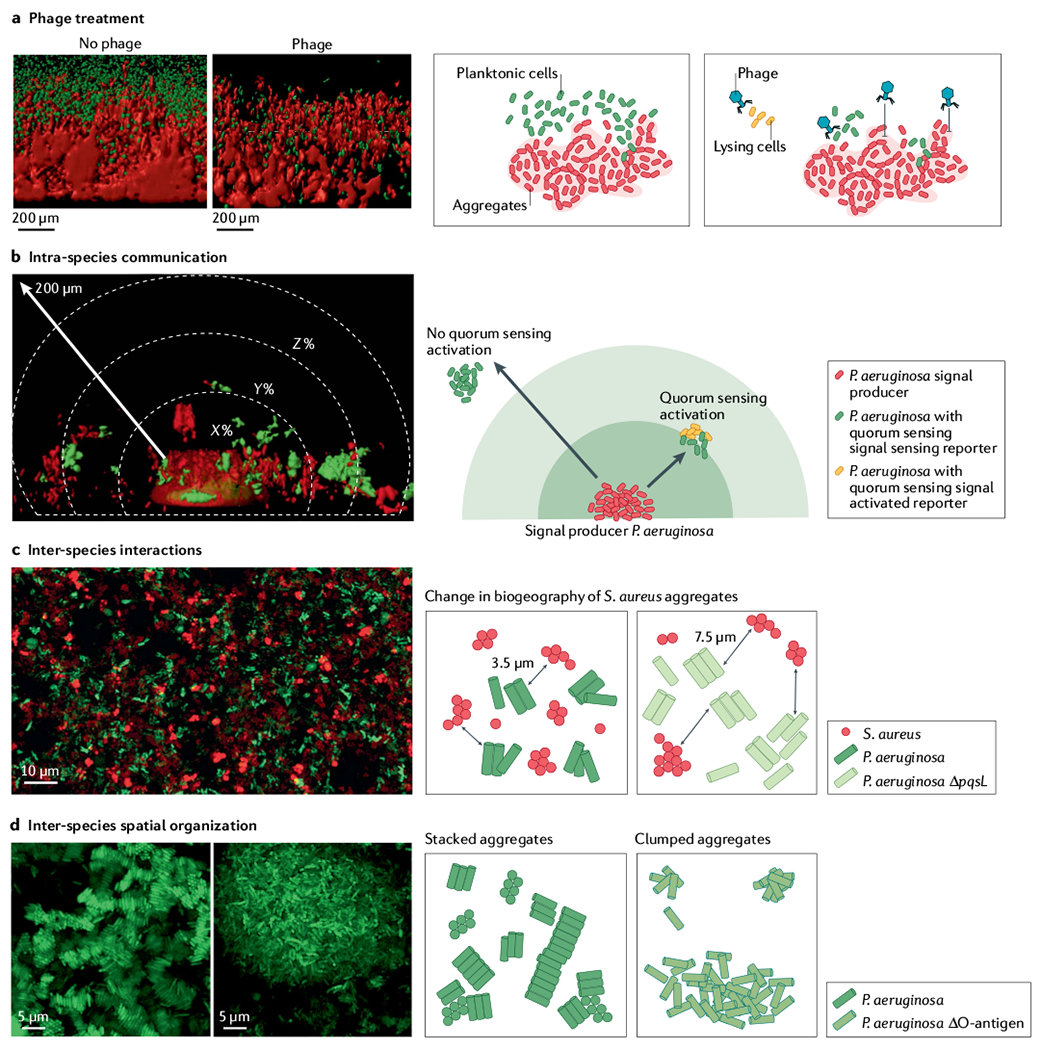
Synthetic cystic fibrosis sputum medium (SCFM2) preclinical model of cystic fibrosis sputum has been a leader in linking biogeography with microbial interactions and fitness. a | Assessment of phage treatment efficacy showed that in SCFM2, planktonic migrant cells are sensitive to phage killing, whereas cells in aggregates and encased in exopolysaccharide matrix limit phage attachment and lysis88. b | SCFM2 provides a structured environment to determine that a Pseudomonas aeruginosa aggregate of ~5,000 cells can signal to neighbouring aggregates up to 176 μm away, although the response to quorum sensing signalling can be heterogeneous85,131. In this experiment, only the aggregate in the bottom centre, confined in a micro-3D-printed trap, can produce the quorum sensing signal. When surrounding aggregates sense the quorum sensing signal, they express a fluorescent reporter gene (red in microscopy image, orange in schematic view). The quorum sensing signal is not detected in surrounding aggregates shown in green. c | Spatially structured environment of SCFM2 enables P. aeruginosa and Staphylococcus aureus aggregates to coexist, unlike in well-mixed environments. In this model, S. aureus aggregates are enriched at a distance of 3.5 μm from P. aeruginosa aggregates. S. aureus fitness did not change when co-cultured with the P. aeruginosa ΔpqsL mutant, which does not produce an anti-staphylococcal molecule. However, the average distance between S. aureus and P. aeruginosa ΔpqsL aggregates increased to 7.6 μm and antibiotic susceptibility of S. aureus increased, showing how changes in inter-species interactions and biogeography can impact S. aureus survival during infection89. d | Loss of O-antigen is a common adaptation of P. aeruginosa to cystic fibrosis airways. This adaptation leads to changes in cell surface hydrophobicity, altering spatial patterning of cells in SCFM2. Cells with O-antigen are stacked, whereas those lacking O-antigen assemble into clumped aggregates91, indicating that genetic variants of P. aeruginosa in cystic fibrosis airways can alter the biogeography of infection. Panel a adapted from REF.88, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Panel b reprinted from REF.85, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Panel c adapted from REF.89, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Panel d reprinted from REF.91, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
