Figure 4. Targeted TNFαIL2 cytokine treatment of solid tumours.
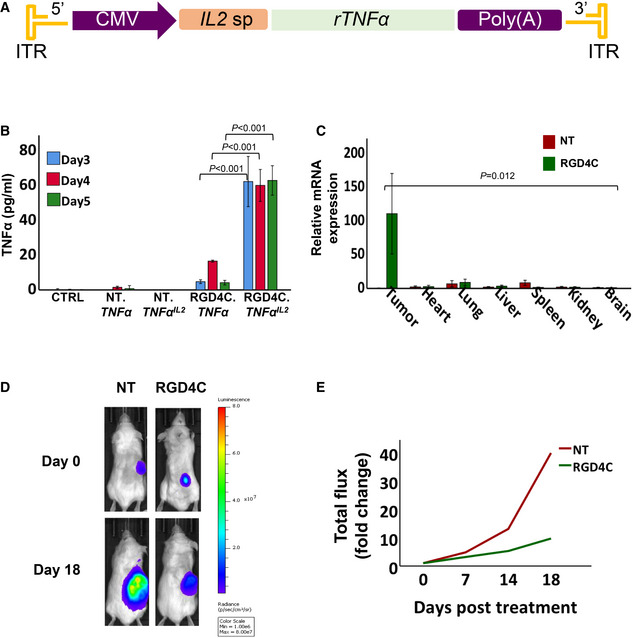
-
ATPA particle DNA carrying the sequence encoding a secreted human soluble TNFα, whose secretion is controlled by the human IL2 signal peptide (sp).
-
BELISA quantification of TNFα production in media collected on days 3 to 5 post‐transduction of human primary GBM001 tumour spheres at 1 × 106 TU/cell of targeted (RGD4C) or non‐targeted (NT) TPA particles carrying native TNFα or recombinant TNFα IL2 sequences. Untreated cells were included as control (CTRL). Data are representative of one experiment and expressed as mean ± SEM. Experiments were repeated twice in triplicates, one‐way ANOVA with Tukey's HSD test was used for data analysis.
-
CBiodistribution of gene delivery upon intravenous administration of 5 × 1010 TU of NT or RGD4C.TPA.TNFα IL2 to NOD/SCID mice bearing subcutaneous human GBM001. Tumours and healthy organs were harvested at day 18 post‐transduction and analysed using RT‐qPCR to quantify the TNFα IL2 transcripts. Data shown are expressed as mean ± SEM, from n = 3 mice. Two‐way ANOVA was used for data analysis.
-
DBLI of Luc showing representative mice with subcutaneous GBM001 labelled with Luc gene, at day 18 post‐targeted RGD4C.TPA.TNFαIL2 treatment.
-
EAnalysis of tumour growth in representative mice, from D, using the tumour bioluminescence data (total flux) and expressed as fold changes of tumour luminescence flux at different time points compared to day 0.
Source data are available online for this figure.
