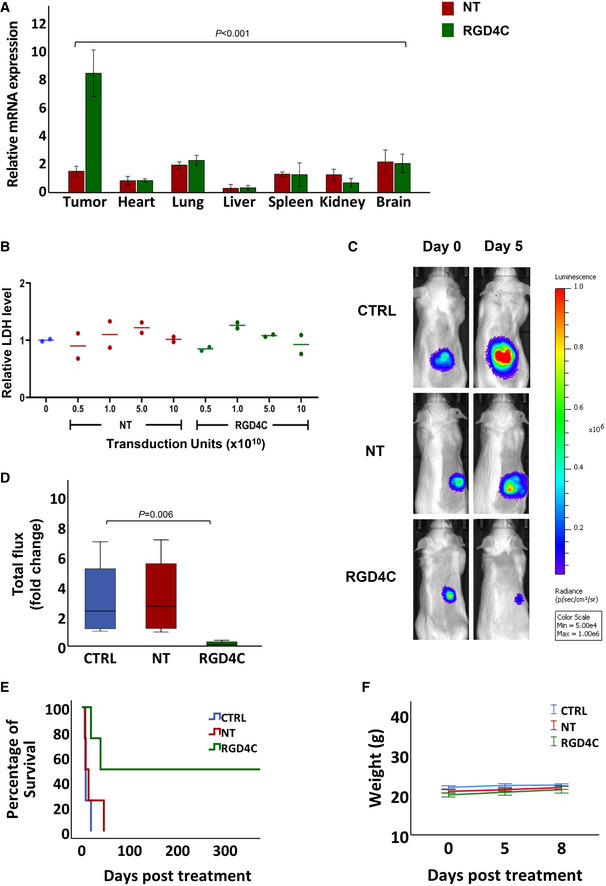
Figure 6. Biodistribution and targeted systemic cancer immunotherapy with RGD4C.TPA.IL15 IgK .
-
ATumour‐bearing BALB/c mice with established subcutaneous CT26.CL25 tumours were intravenously injected with a single dose, 5 × 1010 TU/mouse, of targeted (RGD4C) or non‐targeted (NT) TPA.IL15 IgK . Gene delivery was evaluated by quantifying the IL15 IgK mRNA expression in tumours and healthy tissues at day 5 post‐TPA administration. Data are representative of one experiment, n = 3, and expressed as mean ± SEM. Two‐way ANOVA test was used for data analysis.
-
BSafety of dose escalation regimens assessed at day 5 following TPA delivery, by evaluating the serum levels of LDH from n = 2 mice/TPA dose of targeted (RGD4C) or non‐targeted (NT) TPA.IL15 IgK . Serums from untreated mice were also analysed.
-
CRepresentative tumour‐bearing mice showing bioluminescence imaging of luciferase on days 0 and 5 post‐administration with 5 × 1010 TU of RGD4C.TPA.IL15 IgK (RGD4C) or non‐targeted (NT) particles. The CT26.CL25 tumour cells stably express a luciferase Luc reporter gene. Untreated mice were also included as controls (CTRL).
-
DChanges in tumour viability in mice (n = 4) from day 0 to day 5 post‐TPA administration. Tumour viability was measured by bioluminescence activity. The central band represents the median of the data. The boxes upper and lower lines represent quartile 3 and quartile 1 of the data respectively. The whiskers represent the maximum and minimum outliers of the data. Nonparametric Kruskal–Wallis test was used for data analysis.
-
EKaplan–Meier curves showing survival benefit for tumour‐bearing mice (n = 4) from all experimental groups.
-
FAnimal weights (n = 4) were monitored during therapy experiments and expressed as mean ± SEM.
Source data are available online for this figure.
