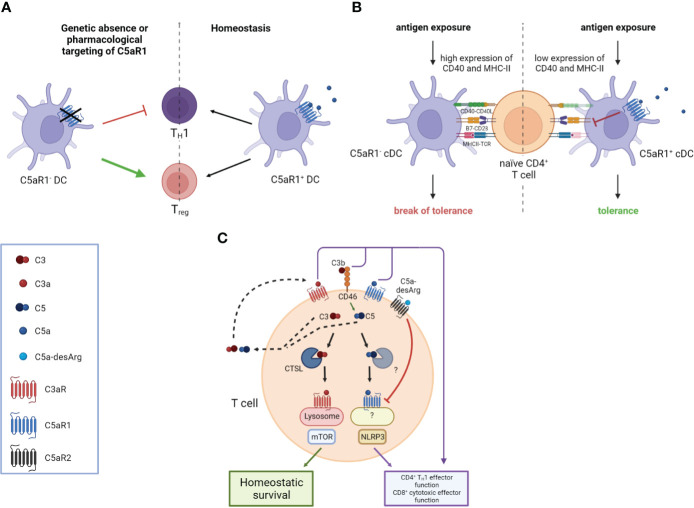
Figure 1.
Impact of the anaphylatoxins on DC-mediated and intrinsic T cell activation. (A) The genetic absence or pharmacological targeting of C5aR1 on DCs leads to attenuated T helper type 1 (TH1) immunity and an increased frequency of Regulatory T cells (Tregs). (B) C5aR1 signaling on naïve mucosal conventional DC2 (cDC2) controls the expression of CD40 and MHC-II which determines the threshold of naïve CD4+ T cell activation. Mucosal antigen exposure is associated with decreased C5aR1 expression; the lack of C5aR1 expression in cDC2s releases the break on CD40 and MHC-II expression resulting in strong CD4+ T cell proliferation and the break of mucosal tolerance. (C) T cell activation triggers the secretion of preformed C3 and C5 into the extracellular space, which can be cleaved into C3a, C3b, C5a, and C5b by canonical and non-canonical mechanisms. Binding of these complement fragments to their respective receptors on the T cell induces CD4+ TH1 and CD8+ effector T cell functions. C3 and C5 are also processed intracellularly by proteases such as cathepsin L (CTSL) in the case of C3 and an unknown protease in the case of C5, respectively. Intracellular C3a is critical to maintain low-level mechanistic target of rapamycin (mTOR) activity by binding to C3aR on lysosomes, thereby contributing to the homeostatic survival of CD4+ T cells. The cleavage of intracellular C5 into C5a and C5b is enhanced by CD46-mediated signaling. C5a engages C5aR1 triggering NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome assembly, eventually driving TH1 differentiation of CD4+ T cells and CD8+ effector T cell functions. Importantly, autocrine engagement of surface-expressed C5aR2 by C5a-desArg can control intracellular C5aR1 activity. Created in BioRender.com.