Figure 3.
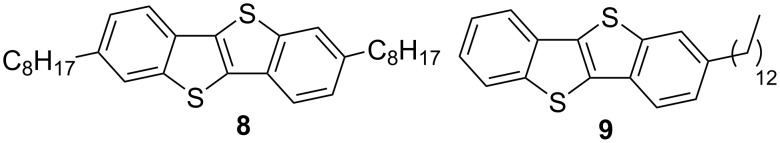
With crystalline films of 2,7-dioctyl[1]benzothieno[3,2-b][1]-benzothiophene (8), obtained by off-centre spin-coating, Bao et al. could obtain remarkable OFET hole mobilities of up to 43 cm2 V−1 s−1 [27]. An asymmetric analogue, which is only alkylated on one side (9), achieved OFET hole mobilities up to 17.22 cm2 V−1 s−1 in polycrystalline films obtained by thermal evaporation [28]; both examples prove the potential of thienoacenes in OFETs.