Figure 1.
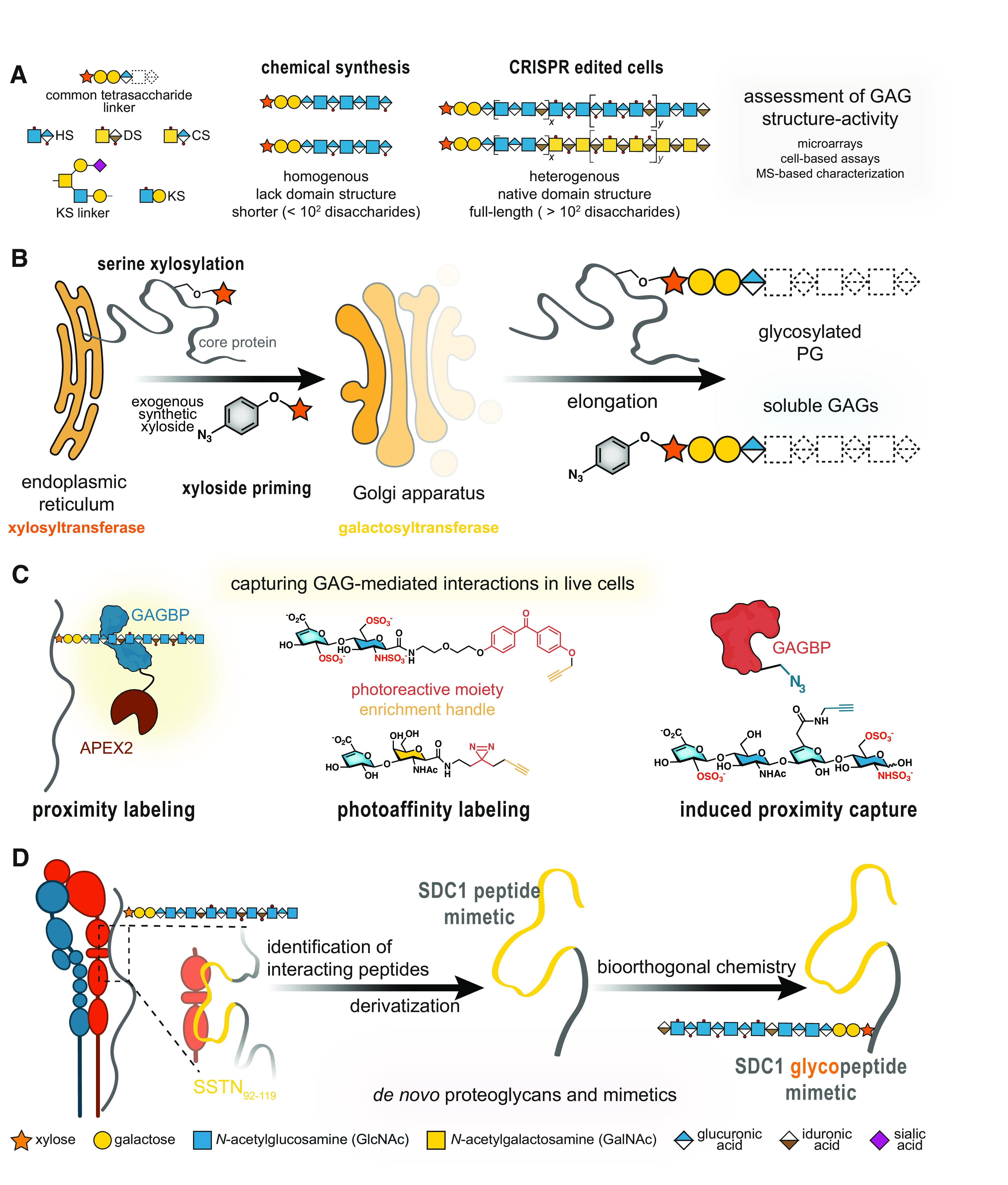
Modern chemical biology approaches to investigate proteoglycan biology. A: chemoenzymatic and total synthesis strategies result in homogenous yet short GAG oligosaccharides, which do not span the lengths or recapitulate the heterogeneity and domain structure of native GAGs. B: synthetic xylosides (bottom) can hijack GAG biosynthesis by mimicking xylosylated core proteins and serving as a substrate for the β4GalT7 galactosyltransferase for elongation. These xylosides can be functionalized with chemical moieties [i.e., azide (2, 3)] for biorthogonal conjugation to surfaces and/or proteins. C: PGs, GAG-binding proteins (GAGBPs), and GAG disaccharides can be modified to enable capture of interaction partners. Proximity labeling with enzymes (e.g., APEX2) tags proteins within a short radius, capturing both direct and indirect interactors. Appending a photoreactive moiety [i.e., benzophenone (top) or diazirine (bottom)] captures interactors of proteins or GAGs (depicted). Induced proximity capture, which requires the modification of both the GAG and associated GAGBP, permits cross linking of GAG to GAGBP complexes. D: polypeptide mimics, such as those derived from the syndecan-1 peptide SSTN92-119 (yellow), permit access to PG glycoconjugates via covalent attachment with GAG oligosaccharides. GAGs, glycosaminoglycans; GAGBPs, GAG-binding proteins; PGs, proteoglycans; SDC1, syndecan-1.
