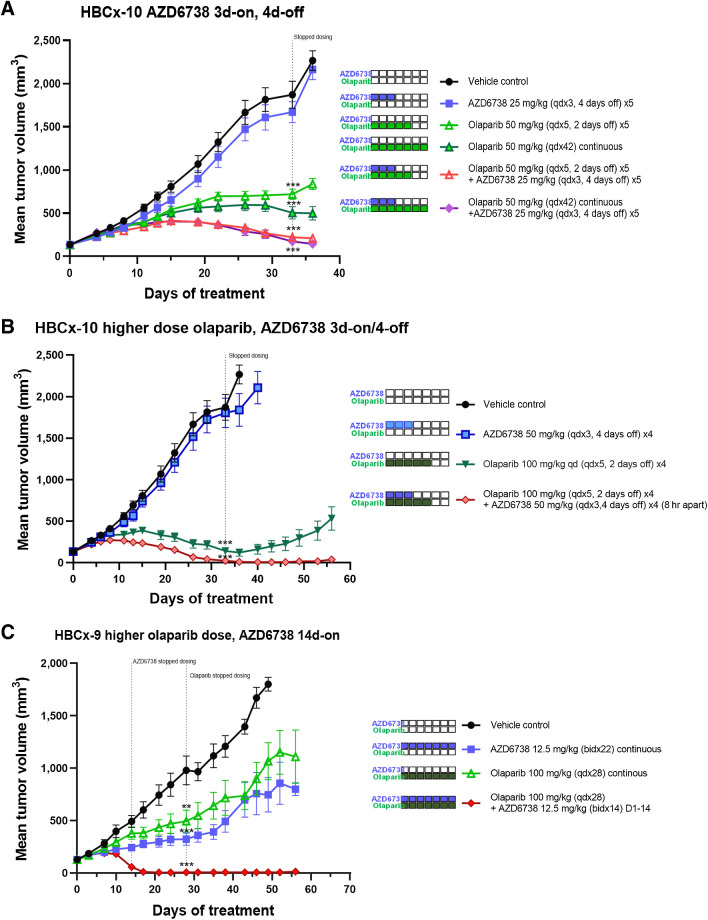
Figure 6.
AZD6738 combination with the PARP inhibitor olaparib efficacy using alternative dose schedules. A, HBCx-10 BRCA-mutant TNBC PDX efficacy when AZD6738 is dosed 3 days-on/4 days-off in combination with low-dose olaparib either on a 5 days-on/2 days-off or continuous daily dosing backbone. B, HBCx-10 BRCA-mutant TNBC PDX when AZD6738 is dosed on 3 days-on/4 days-off in combination with high-dose olaparib on a 5 days-on/2 days-off schedule. C, HBCx-9 BRCA WT TNBC PDX model efficacy when low-dose AZD6738 is dosed twice daily in combination with high-dose olaparib on continuous daily schedule. Mean tumor volume ± SEM is shown. **, P ≤ 0.01; ***, P ≤ 0.001.