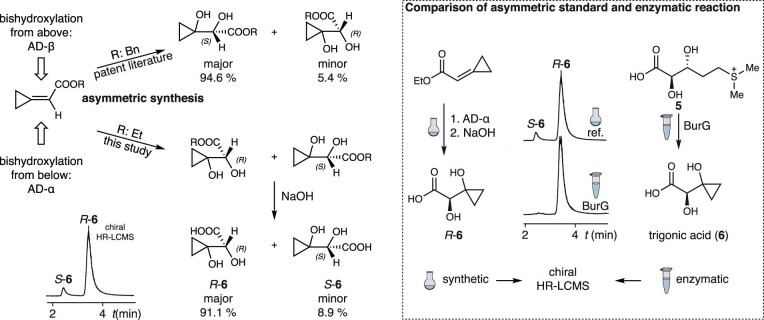
Extended Data Fig. 4. Determination of stereochemistry of trigonic acid (6).
Left panel: stereoselectivity of the asymmetric dihydroxylation (AD)-mix β leads to mainly S-configurated products20; Bn: benzyl protecting group. Consequently AD-mix α forms mainly R-configurated products. Chiral HR-LCMS (bottom left) validates the successful asymmetric synthesis with AD-mix α after hydrolysis of the formed ethyl ester (Et). Right panel: chiral HR-LCMS of asymmetric synthesis products (ref.) vs. enzymatically formed 6 shows the stereochemistry of enzymatically formed 6 to be of R-configuration.