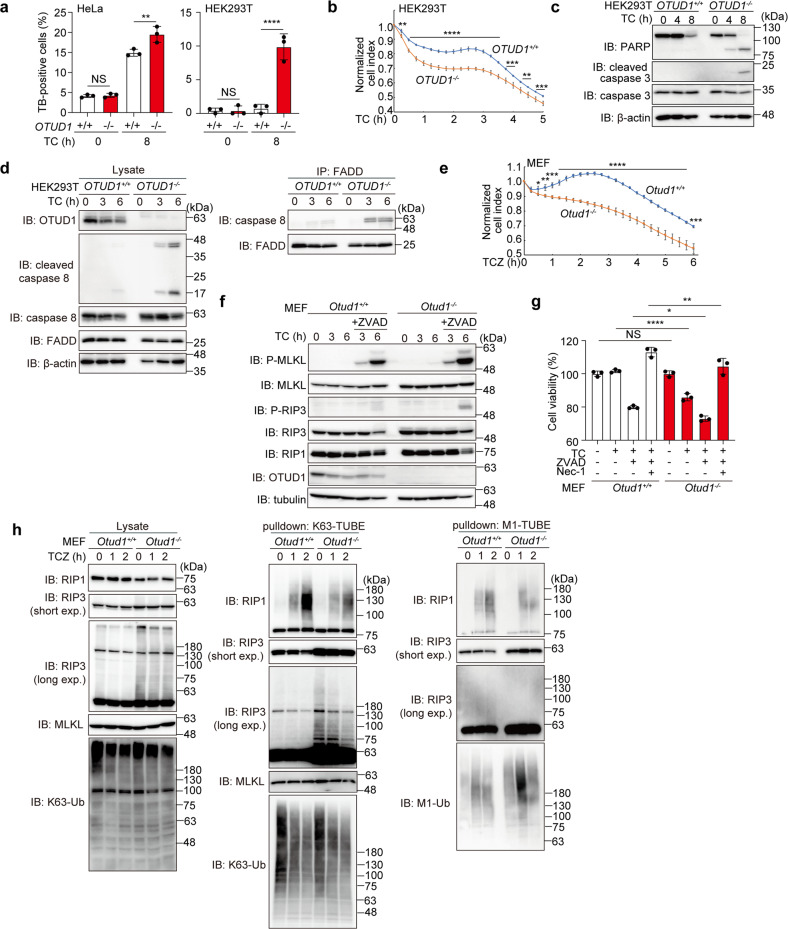
Fig. 4. OTUD1 regulates TNF-α-induced apoptosis and necroptosis.
a Increased TNF-α-induced cell death by the genetic ablation of OTUD1. Wt- and OTUD1−/−-HeLa and HEK293T cells were treated with or without 10 ng/ml TNF-α and 10 μg/ml CHX (TC), and trypan blue-positive cells were counted. b Reduced cell viability of OTUD1-deficient cells. Wt- and OTUD1−/−-HEK293T cells were treated with TC, and the cell proliferation was measured by xCELLigence real-time cell monitoring. c Enhanced apoptosis in OTUD1−/−-HEK293T cells. Wt- and OTUD1−/−-HEK293T cells were treated with TC, and cell lysates were immunoblotted with the indicated antibodies. d TNFR complex II formation was accelerated in OTUD1−/−-HEK293T cells. Wt- and OTUD1−/−-HEK293T cells were treated with TC as in (c). The cell lysates and anti-FADD immunoprecipitates were then blotted with the indicated antibodies. e Increased necroptosis in Otud1−/−-MEFs. Otud1+/+- and Otud1−/−-MEFs were treated with 10 ng/ml TNF-α, 10 μg/ml CHX, and 20 μM ZVAD (TCZ), and the cell proliferation were measured as in (b). f Increased phosphorylation of MLKL and RIP3 in TCZ-treated Otud1−/−-MEFs. MEFs were treated with TC with or without ZVAD as indicated, and the cell lysates were immunoblotted with the depicted antibodies. g Necrostatin-1 (Nec-1) rescued cell death. Otud1+/+- and Otud1−/−-MEFs were treated with TC, ZVAD, and/or 100 μM Nec-1 for 8 h, as indicated, and cell viability was analyzed by a Celltiter Glo assay. h Enhanced K63-ubiquitination of RIP3 in Otud1−/−-MEFs. MEFs were treated with TCZ for the indicated periods, and cell lysates and K63- or M1-TUBE precipitates were immunoblotted with the indicated antibodies. Data are shown as mean ± SD by ANOVA post-hoc Tukey test (a, g; n = 3) or t-test at each time point (b, e; n = 4). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, NS not significant.