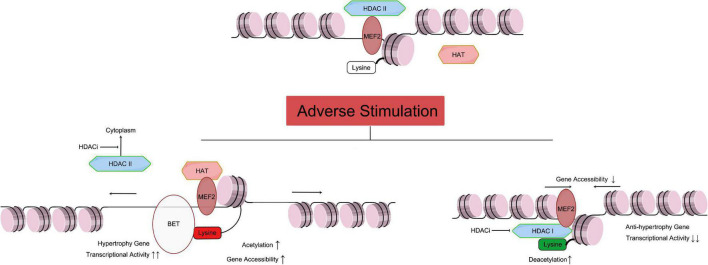
FIGURE 1.
Relationship of HAT and HDAC mediated histone acetylation/deacetylation leading to cardiac hypertrophy. As mentioned above, the Class IIa HDACs are not capable of deacetylating histone residues due to a within the catalytic domain mutation. Therefore, HDAC IIa represses gene transcription by binding with MEF2, recruiting other transcriptional repressors and epigenetic regulators to DNA promoter regions, and maintaining the acetylation level of histone (Figure 1 top part). Under adverse stimulation such as pressure overload, HDAC IIa isolate from MEF2 and transport to cytoplasm, while MEF2 recruiting HAT and catalyze histone lysine residue, and regulate transcription activity. Meanwhile, BET family recognize the acetylation of histone, and bind to related gene promoter region, and promote cardiac hypertrophy (Figure 1 left bottom part). Meanwhile, Class I and IIb HDACs catalyze the removal of acetyl groups from key lysine residues within histone. Histone deacetylation induces chromatin condensation, which represses gene transcription by making gene promoter and enhancer regions less accessible to transcription. Overexpression of HDAC I reduces acetylation of lysine in histone (such as H3K27ac), which will reduce anti-hypertrophy gene transcriptional activity, leading to cardiac hypertrophy. HDACi can inhibit HDAC I catalyze activity, and stop the transport of HDAC II from nuclear to cytoplasm, and protect the heart. MEF, myocyte-specific enhancer; HAT, histone acetyltransferase; HDAC, histone deacetyltransferase; HDACi, HDAC inhibitor. [By Figdraw (www.figdraw.com)].