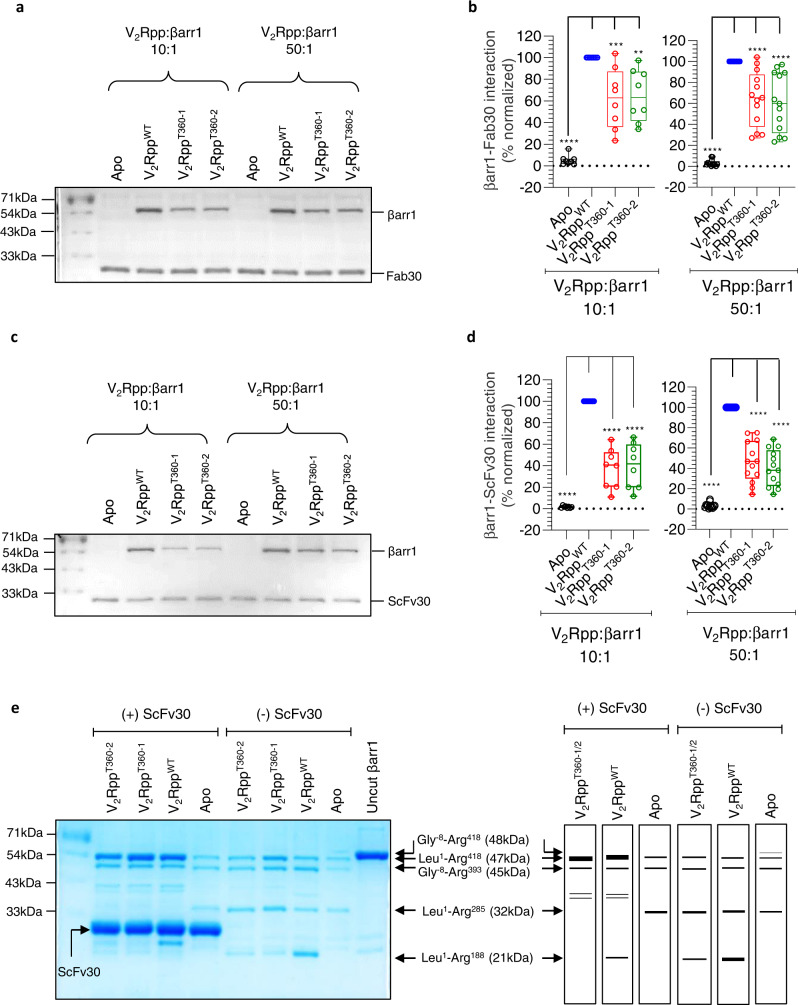
Fig. 2. Recognition of V2RppT360-induced βarr1 conformation by Fab30/ScFv30.
a−d. Fab30 and ScFv30 recognize βarr1 conformation induced by V2RppT360 albeit less efficiently than that induced by V2RppWT. Purified βarr1 was incubated with the indicated phospho-peptides and Fab30/ScFv30 followed by co-immunoprecipitation (co-IP) using Protein L agarose and visualization using Coomassie-stained SDS-PAGE. A representative gel from eight to thirteen independent experiments is shown here. Panels b and d show densitometry-based quantification of βarr1-Fab30/ScFv30 interaction normalized with V2RppWT-βarr1 control (taken as 100%). The data is represented as box plots showing median, IQR with whiskers of 1.5× IQR, and circles representing values from independent experimental replicates. (One-way ANOVA, Dunnett’s multiple comparisons test). The exact p values in 2b are as follows: for (V2Rpp:βarr1 10:1) Apo (p < 0.0001), V2RT360-1 (p = 0.001), V2RT360-2 (p = 0.0021); for (V2Rpp:βarr1 50:1) Apo, V2RT360-1, V2RT360-2 (p < 0.0001); The exact p values in 2d are as follows: for (V2Rpp:βarr1 10:1); Apo, V2RT360-1, V2RT360-2 (p < 0.0001); for (V2Rpp:βarr1 50:1) Apo, V2RT360-1, V2RT360-2 (p < 0.0001); Source data are provided as a Source Data file (**p < 0.01 ***p < 0.001, ****p < 0.0001). e Binding of ScFv30 influences limited proteolysis pattern of βarr1 for the wild-type and mutant phospho-peptides similarly (30 min). βarr1 activated with 50-fold molar excess of indicated phospho-peptides was subjected to limited trypsin proteolysis at a trypsin:βarr1 ratio of 1: 50 in the presence or absence of ScFv30 followed by visualization of the bands on SDS-PAGE. A representative gel from four independent experiments (left panel) and a schematic of proteolysis patterns (right panel) are shown here. Source data are provided as a Source Data file.