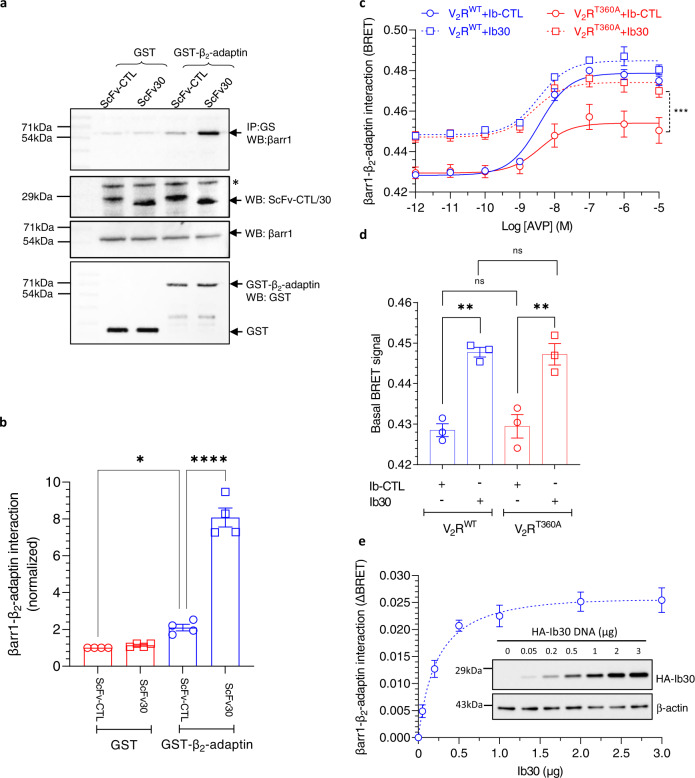
Fig. 9. Intrabody30 enhances the interaction of β2-adaptin with βarr1.
a Purified GST-β2-adaptin (592–951) was incubated with V2RT360A and βarr1 in presence of ScFv-CTL or SvFv30 followed by co-IP and Western blotting. Unconjugated GST was used as a negative control. A representative blot from three different experiments is shown here. The * symbol designates a non-specific band that we typically observe in lysates prepared from Sf9 cells. b Densitometry-based quantification (mean ± SEM) of βarr1-β2-adaptin interaction from four independent experiments normalized with GST control (One-way ANOVA, Sidak’s multiple comparisons test; *p = 0.0308, ****p < 0.0001). c BRET between RlucII-tagged βarr1 and YFP-tagged β2-adaptin shows enhanced interaction between βarr1 and β2-adaptin in presence of Ib30, as compared to Ib-CTL, for both V2RWT and V2RT360A. Data (mean ± SEM) from three independent experiments (Two-way ANOVA, Tukey’s multiple comparisons test; ***p = 0.0006) are presented here. d The BRET signal at lowest ligand concentration under different conditions as measured in panel C from three independent experiments (mean ± SEM; One-way ANOVA, Tukey’s multiple comparisons test; V2RWT (p = 0.0012); V2RT360A (p = 0.0020), ns = non-significant). e Ib30 induced increase in βarr1-β2-adaptin interaction exists even in the absence of either V2RWT and V2RT360A. βarr1-β2-adaptin interaction in presence of Ib30 exhibits a concentration-dependent increase until saturating concentration of the latter. Data represent four independent experiments (mean ± SEM). The inset shows a representative blot indicating the concentration range of Ib30 used in the BRET experiment, expression level and loading control (β-actin). Source data are provided as a Source Data file.