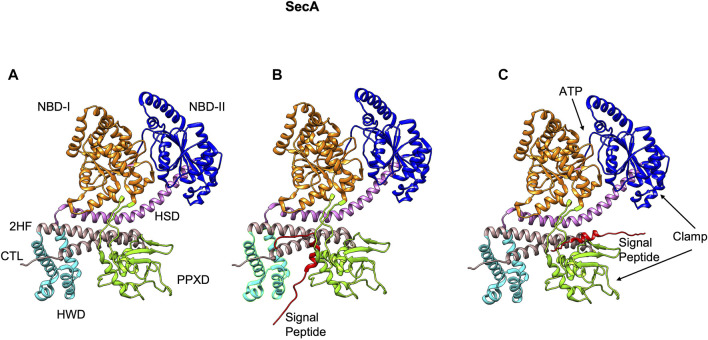
FIGURE 5.
The NMR structure of SecA from E. coli [adapted from Gelis et al. (2007) PDB: 2VDA]. (A) The various domains of SecA are highlighted (without the signal peptide). The nucleotide binding domains I (orange) and II (blue), the central helix subdomain of helical scaffold domain (HSD in purple), the preprotein crosslinking domain (PPXD green), the helical wing domain (HWD cyan), and the observed carboxyl-terminal linker domain (CTL). Also highlighted is the 2-helix finger (2HF tan) within the HSD domain. (B) The signal peptide (red) binds roughly perpendicular to 2HF based on NMR studies (Gelis et al., 2007). (C) The signal peptide is modeled parallel to the 2HF of the E. coli SecA NMR structure based on FRET, mutagenesis and genetic studies (Grady et al., 2012).