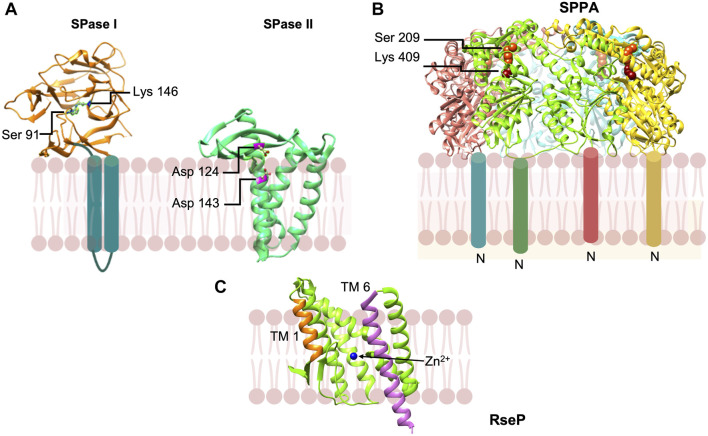
FIGURE 9.
Peptidases involved in the removal of signal peptides and their degradation. (A) Signal peptidase 1 [adapted from Paetzel et al. (2004) PDB: 1T7D] is a novel Ser-Lys protease that cleaves the preprotein at the membrane surface on the periplasmic side. Signal peptidase 2 [adapted from Vogeley et al. (2016) PDB: 5DIR] is an aspartic acid protease that cleaves a diacyl glyceride modified preproteins within the plane of the membrane. (B) SppA [adapted from Kim et al. (2008) PDB: 3BF0] is a tetrameric protein that degrades signal peptides which are released from the membrane into the periplasmic space. SppA employs a Ser-Lys dyad and is anchored to the membrane by an amino terminal TM segment. (C) RseP signal peptide peptidase [from Methanocaldococcus jannaschii adapted from Feng et al. (2007) PDB: 3B4R] in open state. Both water molecules and peptide substrates reach the active site containing Zn2+ ion (blue) during its open state.