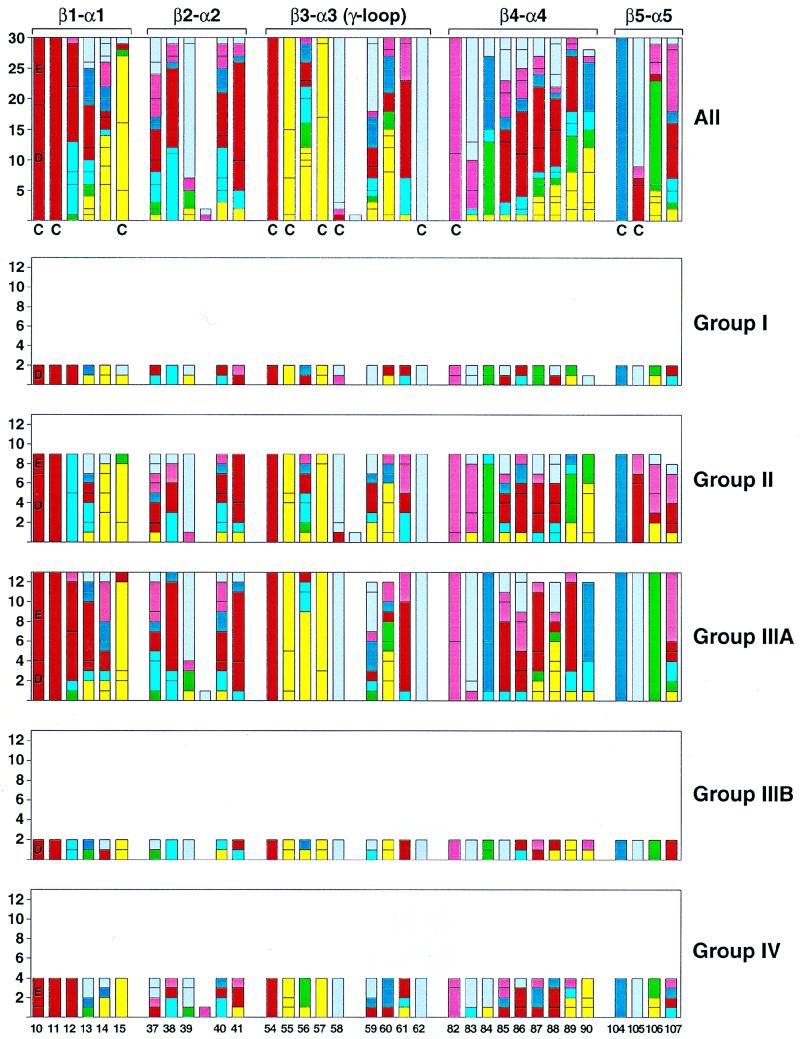
FIG. 7.
Sequence alignment of response regulator loop regions proximal to the site of phosphorylation for B. subtilis. The number of each response regulator residue type at loop positions spanning between secondary structure elements β-strand 1 to α-helix 1 (β1-α1), β2-α2, β3-α3, β4-α4, and β5-α5 are illustrated individually as members of groups I, II, IIIA, and IIIB, IV and collectively in the top bar graph for comparison. The residue type is coded by color: acidic in red (D and E), basic in dark blue (K and R), hydroxyl in rose (S and T), polar in light blue (H, N, and Q), hydrophobic in yellow (C, I, L, V, and M), aromatic in green (Y, F, and W), and structural in gray (A, G, and P). The numbering scheme is based on the Spo0F sequence, and conserved residues essential for phosphorylation are D10, D11, D54 (the phosphorylation site), T82, and K104 by this numbering. Residues previously described as conserved (C) by alignment of response regulators from all organisms (30) are denoted. Response regulator sequences (14) were aligned with Clustal W (10) and illustrated with the Excel 98 (Microsoft) and Adobe Illustrator programs (Adobe).