Abstract
EFSA received a mandate from the European Commission to assess the effectiveness of prohibitions of certain activities in restricted zones, and of certain risk mitigation treatments for products of animal origin and other materials with respect to diseases included in the Category A list in the Animal Health Law (Regulation (EU) 2016/429). This opinion belongs to a series of opinions where other disease‐specific control measures have been assessed. In this opinion, EFSA and the AHAW Panel of experts review the effectiveness of (i) prohibiting the movements of certain products, notably germinal products (semen, oocytes, embryos and hatching eggs), products of animal origin and animal by‐products and feed of plant origin, hay and straw, and (ii) risk mitigation treatments for products of animal origin. In terms of semen, oocytes, embryos and hatching eggs, it was agreed that there was a lack of evidence particularly for embryos and oocytes reflected in a varying degree of uncertainty, whether these commodities could potentially contain the pathogen under consideration. The scenario assessed did not consider whether the presence of pathogen would lead to infection in the recipient animal. In terms of animal products, certain animal by‐products and movement of feed of plant origin and straw, the assessment considered the ability of the commodity to transmit disease to another animal if exposed. For most pathogens, products were to some degree considered a risk, but lack of field evidence contributed to the uncertainty, particularly as potential exposure of ruminants to meat products is concerned. In terms of the risk mitigating treatments, recommendations have been made for several of these treatments, because the treatment description is not complete, the evidence is poor or inconclusive, or the evidence points to the treatment being ineffective.
Keywords: Category A diseases, control measures, risk‐mitigating treatments, movement prohibitions, germinal products, animal products
Summary
This opinion is the last in a series of opinions of a mandate received from the European Commission to consider control measures for Category A diseases. The background and specific details of this mandate can be found in the opinion. Specifically, this opinion assesses the prohibitions of certain movements in restricted zones and risk mitigation treatments for products of animal origin. The methodology used in this series of opinions, covering all Category A diseases, was agreed on, and published in a separate technical report.
The EFSA AHAW Panel and its working group agreed that irrespective of the purpose or nature of the activity, it is recommended to not move animals from restricted zones without appropriate mitigation measures. Such measures can be found in the earlier opinions of this series. There is a lack of evidence to allow making conclusions with high degree of certainty of the possibility that germinal products collected or derived from an infected animal of the listed species (i.e. kept animals, and, where relevant, game animals) in the restricted zone can contain the disease agent, particularly regarding oocytes, in vivo derived and in vitro produced embryos. Specifically, for African horse sickness (AHS), African swine fever (ASF), highly pathogenic avian influenza (HPAI), Newcastle disease (ND) and lumpy skin disease (LSD), evidence suggests virus may be present in semen and for foot and mouth disease (FMD) in oocytes, and while no data has been identified to suggest transmission is not possible, there are currently no prohibitions on the collection of semen for AHS, HPAI and ND. Although internationally accepted guidelines exist (e.g. IETS), no standardised rules for the safe collection of germinal products for domestic use exist. Their adoption also for domestic use could potentially reduce the possibility that a disease agent is present in the germinal products after their collection.
There is a lack of evidence from observational studies in endemic areas to allow making conclusions with high degree of certainty on the role of animal products, animal by‐products and feed of plant origin and straw in epidemics. The assessment of the risk associated with movements of meat products has a large degree of uncertainty due to the large variation of production processes and the likelihood of exposure to susceptible animals.
In general, the observed uncertainty related to the assessments of the possibility that the disease agents can be spread via the movement of feed is due to the wide range of different feed materials and the lack of scientific evidence on survival of the disease agents in these. It was concluded that a particular risk is associated with local movements of feed material of plant origin and straw obtained in the protection zone that has been contaminated by infected livestock, and, where relevant, by infected wildlife, during production or storage.
Recommendations were made to address both lack of evidence and contradictory evidence using well‐designed scientific studies in certain areas. Further studies for the safe trade of germinal products with respect to several pathogens is required before they should be traded from restricted zones, even though they are not currently prohibited. Conversely, regarding FMD, prohibitions regarding movements of in vivo derived embryos of cattle could be reconsidered. Scientific evidence is required to conclude that the movement of the animal products, animal by‐products and movements of feed of plant origin and straw would be safe, with the exception of movements from an AHS control zone. Observational studies and outbreak investigations should be used to provide better evidence about the role products play in disease spread. For the case of AHS, while it was considered extremely unlikely that movements of products would lead to disease spread, there is very little evidence to confirm this. The experts agreed that while the evidence has been presented for zoonotic diseases only based on animal‐to‐animal transmission, the public health risk should also be considered. In terms of risk mitigating treatments, experts recommend descriptions of heat treatments should consider the core temperature and the duration to maintain the temperature at the core to be effective, and similarly, for treatments involving a change of pH, the pH that must be reached throughout the treated product and the duration for which that pH needs to be maintained to be effective should be provided. Should no scientific evidence exist for the missing information or where the treatment is assessed as inconclusive, it is recommended to carry out experimental studies to fill the knowledge gaps. However, where treatments have been assessed as not effective based on the identified scientific evidence, the Panel recommends not to use them. Where available, alternative treatments identified through the extensive literature search that have been assessed as effective for the disease agent should be applied.
It was also recommended that risk managers consider the licensing for movements of feed material and straw out of a protection zone for other diseases than just RP and FMD. For the mitigation of the foot and mouth disease virus (FMDV) spread risk associated with feed materials of plant origin and straw, it is recommended to monitor that the temperature of 80°C is reached for 10 min throughout the material when applying ‘Heat treatment, minimum temperature of 80°C and for a minimum of 10 min, steam in a closed chamber’. It is recommended to apply the storage period of 4 months stipulated by WOAH (formerly OIE) for storage of feed materials of plant origin and straw in package or bales. For those additional Category A diseases for which feed materials of plant origin and straw pose a risk of spread, including through vectors, it is recommended to assess the effectiveness of risk mitigation treatments.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Regulation (EU) 2016/429 on transmissible animal diseases (‘Animal Health Law’), hereinafter referred to as AHL, requires the Commission to lay down detailed rules on the disease control measures against listed diseases as referred to in point (a), (b) and (c) of its Article 9 (Category A, B and C diseases). The Commission is empowered to adopt delegated acts supplementing the rules laid down in Part III of Regulation (EU) 2016/429 on transmissible animal diseases (Animal Health Law) on disease control measures for listed diseases as referred to in point (a), (b) and (c) of its Article 9 (Category A, B and C diseases). Therefore, the Commission has developed and adopted a Delegated Regulation 1 laying down rules for the prevention and control of certain diseases (‘the Delegated Regulation’). The rules laid down in the Delegated Regulation are in respect of terrestrial animals largely replicating the rules currently in force concerning the disease control measures in the event of animal diseases with serious effects on the livestock as they have proven to be effective in preventing the spread of those diseases within the Union. Consequently, many animal disease control measures laid down in existing Directives will be, to the extent that not already done by the Animal Health Law, replaced by the rules provided in the Delegated Regulation. At the same time, these rules have been aligned with the international standards from the World Organisation for Animal Health (WOAH), wherever these existed. However, certain disease control measures proposed in the Delegated Regulation, in particular in its Annexes, were considered as outdated, i.e. possibly not based on most recent scientific evidence at the time of development. Their review is considered as necessary. Moreover, for those Category A diseases for which rules were not established before or were not detailed enough, certain disease control and risk mitigating measures are, due to the lack of scientific basis, extrapolated from other diseases, for which rules existed in the past. Finally, for some other diseases the evidence and scientific knowledge, was not available to the Commission and to the Member States at the time of developing the Delegated Regulation due to the time constraints. The following diseases are examples of the later: infection with Rift Valley fever (RVF), infection with Mycoplasma mycoides subsp. mycoides (Contagious bovine pleuropneumonia) (CBPP), Contagious caprine pleuropneumonia (CCPP), Sheep pox and goat pox, infection with peste des petit ruminants virus (PPR), African horse sickness (AHS), Glanders. In this regard, the existing rules will cease to apply as from the date of application of the Animal Health Law and its complementing legislation including the Delegated Regulation, i.e. from 21 April 2021. Certain of the proposed measures for the prevention and control of Category A diseases of terrestrial animals should therefore be assessed in order to ensure that they are effective and updated based on the latest scientific knowledge in this new set of legislation. This is particularly important in the case of those diseases that are less common or have been never reported in the Union.
ToR 1: Sampling of animals and establishments for the detection of Category A diseases in terrestrial animals
Based on available scientific information, assess the effectiveness of existing sampling procedures to detect or rule out the presence of each Category A disease of terrestrial animals and, in case of absence of effective procedures, develop them, in order to complete the rules provided for in Annex I to the Delegated Regulation. In particular, provide for disease‐specific procedures for the sampling of:
ToR 1.1 Animals for clinical examinations to ensure the detection of the relevant Category A disease during the performance of official investigations in establishments that are affected or suspected to be affected by Category A diseases and visits in establishments located in restricted zones in accordance with Articles 6(2), 13(3)(c), 14(1) and 26(2) of the Delegated Regulation.
ToR 1.2 Animals for laboratory examinations to ensure the detection of the relevant Category A disease during the performance of official investigations in establishments that are affected or suspected to be affected by Category A diseases and visits in establishments located in restricted zones in accordance with Articles 6(2), 12(3), 13(3)(c), 14(1), 26(2) of the Delegated Regulation.
ToR 1.3 Establishments to ensure the detection of the relevant Category A disease for the performance of visits in establishments located in protection zones larger than 3 km and establishments located in the surveillance zone in accordance with Articles 26(5) and 41 of the Delegated Regulation.
ToR 1.4 Animals for clinical and laboratory examinations to ensure the detection of the relevant Category A disease for the movement of animals from restricted zones in accordance with Articles 28(5), 43(5), 56(1)(c) of the Delegated Regulation.
ToR 1.5 Animals for laboratory examinations to ensure the detection of the relevant Category A disease before and after being introduced in the affected establishments for repopulation, in accordance with Article 59(2), (3) and (9) of the Delegated Regulation.
ToR 2: Monitoring period
ToR 2.1 Assess the effectiveness of the length of the monitoring periods set out in Annex II of the Delegated Regulation for each Category A disease of terrestrial animals. In this regard, it is important to take into consideration that the monitoring period was introduced as a management tool, which represents a time frame of reference assigned to each Category A disease for the competent authority to apply certain control measures and to carry out investigations in the event of suspicion and confirmation of Category A diseases in terrestrial animals.
This assessment should be carried out with respect to the following situations:
-
a
the records analysis carried out by the competent authority in the framework of the epidemiological enquiry referred to in Article 57 of Regulation (EU) 2016/429, in the event of suspicion of a Category A disease (Article 8(4) of the Delegated Regulation);
-
b
the derogation from killing in the event of an outbreak of a Category A disease in establishments keeping animals of listed species in two or more epidemiological units (Article 13(1) of the Delegated Regulation);
-
c
the tracing carried out by the competent authority to identify establishments and other locations epidemiologically linked to an establishment affected by a Category A disease (Article 17(2) of the Delegated Regulation);
-
d
the exemption applied to certain products from the prohibitions laid down in Annex VI taking into account the date they were produced (Article 27(3)(c) of the Delegated Regulation);
-
e
the specific conditions for authorising movements of semen from approved germinal product establishments in the protection and surveillance zones (Article 32(c) and 48(c) of the Delegated Regulation);
-
f
the repopulation of establishments affected by a Category A disease (Article 57(1)(b) and 59(4)(b) of the Delegated Regulation).
ToR 2.2 Propose the length of what should be the monitoring period in those diseases for which the time is assessed as not effective.
ToR 3: Minimum radius of restricted zones and duration of the disease control measures in restricted zones
ToR 3.1 Assess the effectiveness to control the spread of the disease of the minimum radius of the protection and surveillance zones set out in Annex V of the Delegated Regulation for each Category A disease of terrestrial animals.
ToR 3.2 Assess the effectiveness to control the spread of the disease of the minimum periods during which the competent authority should apply the restriction measures in the protection and surveillance zones as set out in Annex X and XI for each Category A disease of terrestrial animals.
ToR 4: Prohibitions in restricted zones and risk‐mitigating treatments for products of animal origin and other materials
ToR 4.1 Assess the effectiveness to control the spread of disease of prohibitions set out in Annex VI of the Delegated Regulation with respect to the risk associated for each Category A disease, to the listed activities and commodities.
ToR 4.2 Review the available scientific information on risk‐mitigating treatments that are effective to control the presence of Category A disease agents in products of animal origin and other relevant materials. Based on this:
-
a
provide an opinion on the effectiveness of the risk‐mitigating treatments for products of animal origin and other materials produced or processed in the restricted zone set out in Annex VII and VIII, and
-
b
if relevant, suggest new treatments or procedures that can be effective to mitigate or to eliminate such risk.
1.2. Interpretation of the Terms of Reference
ToRs 1, 2 and 3 have been addressed in 14 individual opinions, one for each of the Category A diseases for terrestrial animals (EFSA AHAW Panel, 2021a, 2021b, 2021c, 2021d, 2021e, 2021f, 2021g, 2021h, 2022a, 2022b, 2022c, 2022d, 2022e, 2022f).
This document addresses ToR 4 that focusses on the prohibitions in restricted zones and risk‐mitigating treatments for products of animal origin and other materials listed in Annexes VI, VII and VIII of the Delegated Regulation.
1.2.1. Problem formulation
ToR 4.1 concerns Annex VI of the Delegated Regulation (DR). This annex, as referred to in Art 27 of the DR, lists prohibitions of activities concerning animals of listed species and products from those animals within, from and to the restricted zone (= protection zone and surveillance zone).
Some of the prohibitions concern movements of animals, some concern movements of germinal products (semen, oocytes, embryos and hatching eggs), some concern products of animal origin and feed of plant origin and straw.
Annex VI of the DR lists general prohibitions that result from the confirmed detection of a Category A disease in a European Member State. These prohibitions aim at preventing the spread of the disease. The assessment considers the general pathogenesis and resulting pathology or other negative effects caused by the disease agent in the listed animal species and related matrices that could be contaminated with the disease agent. It aims at identifying, based on the known biological course of the infection, if and through which routes an infected animal sheds the disease agent, and which organs of an infected animal can contain the disease agent.
The epidemiological details and deviations from the general pathology and the resulting possibilities of derogating from the general prohibitions (if and under which circumstances) are the focus of other ToRs of the mandate (1, 2) and have been assessed in other EFSA scientific opinions (EFSA AHAW Panel, 2021a, 2021b, 2021c, 2021d, 2021e, 2021f, 2021g, 2021h, 2022a, 2022b, 2022c, 2022d, 2022e, 2022f).
ToR 4.2 concerns Annexes VII and VIII of the DR, which list several risk‐mitigating treatments for the different disease agent. The treatments aim at inactivating the disease agent in products of animal origin or other materials produced or processed in the restricted zone. The assessment of ToR 4.2 therefore focusses on the susceptibility of the disease agent to different treatments. This includes treatments listed in Annexes VII and VIII and additional treatments described elsewhere and identified through the extensive literature search (ELS) carried out for this assessment to address both ToR 4.2 (a) and (b).
1.3. Translation of the ToRs into assessment questions and sub‐questions
1.3.1. ToR 4.1
ToR 4.1 has been translated into four assessment questions.
The first question regards the movements of animals and investigates if infected animals of listed species (i.e. kept animals, and, where relevant, game animals and wild animals) can transmit the disease agent as a result of the activity listed in Annex VI. For the assessment, it is assumed that no specific mitigating measures have been applied to the animals. Transmission of the disease agent is understood as the infection of another animal with the disease agent by direct or indirect means. The activities assessed with this question are:
-
•
movements of kept animals of listed species from establishments in the restricted zone,
-
•
movements of kept animals of listed species to establishments in the restricted zone,
-
•
restocking of game animals of listed species,
-
•
fairs, markets, shows and other gatherings of kept animals of listed species including collection and dispersion of those species,
-
•
itinerant natural service of kept animals of listed species.
The second question regards movements of germinal products. Regarding hatching eggs, the question investigates if an infectious disease agent can be present in/on hatching eggs of infected animals of listed species. For the assessment, it is assumed that no specific mitigating measures have been applied to the parent flock or the hatching eggs. Regarding semen, oocytes and embryos (in vivo and in vitro produced), the question investigates if the germinal product collected from infected kept animals of listed species can contain the infectious disease agent. It is assumed that no specific mitigating measures have been applied to the germinal products or their donors, and that the germinal products have undergone only routine treatment after their collection, e.g. addition of antimicrobials, if this is routinely done. The activities assessed with this question are:
-
•
movements of hatching eggs from establishments in the restricted zone,
-
•
movements of semen obtained from kept animals of listed species from establishments in the restricted zone,
-
•
collection of semen, oocytes and embryos from kept animals of listed species,
-
•
itinerant artificial insemination of kept animals of listed species,
-
•
movements of embryos (in vivo or in vitro produced) or oocytes (with intact zona pellucida) obtained from kept animals of listed species from establishments in the restricted zone.
The third question regards certain animal products and investigates if the product collected from infected kept (and wild) animals of listed species can contain the infectious disease agent. It is assumed that products have not undergone any specific risk‐mitigating treatment before or after their production. The activities assessed with this question are:
-
•
movements of fresh meat excluding offal from kept and wild animals of listed species from slaughterhouses or game handling establishments in the restricted zone.
-
•
movements of offal from kept and wild animals of listed species from slaughterhouses or game handling establishments in the restricted zone.
-
•
movements of meat products obtained from fresh meat of listed species from establishments in the restricted zone.
-
•
movement of raw milk and colostrum obtained from kept animals of listed species from establishments in the restricted zone.
-
•
movement of dairy products and colostrum‐based products from establishments in the restricted zone.
-
•
movement of eggs for human consumption from establishments in the restricted zone.
-
•movements of animal by‐products from kept animals of listed species from establishments in the restricted zone, except entire bodies or parts of dead animals.
-
•Hides, skins, wool, bristles and feathers.
-
•Animal by‐products other than manure, including litter and used bedding, and other than hides, skins, wool, bristles and feathers.
-
•
The fourth question concerns feed of plant origin, straw and manure, including litter and used bedding, and investigates if the material can be contaminated with the infectious disease agent by infected animals of listed species. It is assumed that the material has not undergone any specific risk‐mitigating treatment. The activities assessed with this question are:
-
•
movement of feed material of plant origin and straw obtained in the protection zone.
-
•
movement of manure, including litter and used bedding from kept animals of listed species from establishments in the restricted zone.
1.3.2. ToR 4.2
ToR 4.2 was translated into two assessment questions. Both assessment questions were applied to the treatments currently listed in Annexes VII and VIII, as well as to additional treatments identified through the ELS, to cover both ToR 4.2 a and b.
The first question regards the products of animal origin and investigates if the treatment listed in Annex VII is effective in inactivating the disease agent. The second question regards the products not of animal origin and investigates if the treatment listed in Annex VIII is effective in inactivating the disease agent.
It has to be noted that it may be either difficult or not possible to make general statements regarding the effectiveness of the treatment as given in the annexes of the DR in the absence of complete information regarding the matrix, the treatment and the disease agent (including its amount present in the matrix). In addition, if scientific evidence on the specific treatment is not available, the assessment of the treatment's effectiveness has to be based on extrapolations based on data available for other related disease agents or treatments and expert opinion, which add to the uncertainty. If the description of the treatments is not sufficiently complete to allow for an assessment with high degree of certainty (i.e. 90–100%) or 0–33% probability (see Section 2.2 on Evidence assessment and uncertainty analysis), a recommendation may be made regarding a clarification of the treatment.
For the assessment of the two questions, it is assumed that all legal requirements foreseen for the respective business as usual food production processes are applied, but that no testing of the animals for the Category A diseases has taken place before the production process.
2. Data and methodologies
2.1. Evidence collection
Scientific evidence for the assessment of animal movements has been collected through an ELS and summaries in the sections ‘Epidemiology’ of earlier EFSA scientific outputs, which also provide details on the ELS protocol applied (EFSA AHAW Panel, 2021a, 2021b, 2021c, 2021d, 2021e, 2021f, 2021g, 2021h, 2022a, 2022b, 2022c, 2022d, 2022e, 2022f).
To collect scientific evidence on the presence of the disease agents in different organ systems of infected animals, an ELS on experimental infections of listed animal species was carried out. Scientific evidence on the survival of the disease agents in different products of animal origin and other products was collected through an ELS on disease agent survival. A further ELS collecting scientific evidence on the presence and survival of the disease agents in semen, oocytes and embryos was carried out. Scientific evidence on treatments to mitigate the risk of the different disease agents has been collected through an ELS. The ELS protocols with the search results and the evidence extracted are available in Annexes Annex A – Extensive literature search on the presence of selected Category A disease pathogens in germinal products, Annex B – Extensive literature search on the presence of selected Category A disease pathogens in animal products, animal by‐products and feed of plant origin and straw–C.
2.2. Evidence assessment and uncertainty analysis
The assessment of the different assessment questions was carried out in a three‐stage expert opinion process in which one Working Group (WG) member was the facilitator and the remaining six WG members participated as experts.
In the first stage, the scientific evidence for each assessment question regarding movements of animals, germinal products, animal products and other materials (ToR 4.1) and risk mitigating treatments for products of animal origin, risk mitigating treatments for products not of animal origin) collected through the ELSs (ToR 4.2 a and b) was collated in evidence dossiers for all disease agents to be assessed and distributed to the experts of the WG.
The WG experts reviewed the evidence and individually answered the assessment question for each disease agent, providing the reasoning for their answer. The answer included the expression of their certainty around the assessment, using a quantitative scale, expressed both numerically and verbally, as proposed in EFSA's Guidance on Uncertainty Analysis in Scientific Assessments (EFSA Scientific Committee, 2018) (Table 1).
Table 1.
Probability scale (expressed in %) used to express certainty about the judgements
| Probability term | Subjective probability range |
|---|---|
| Almost certain | 99–100% |
| Extremely likely | 95 to < 99% |
| Very likely | 90 to < 95% |
| Likely | 66 to < 90% |
| About as likely as not | 33 to< 66% |
| Unlikely | 10 to < 33% |
| Very unlikely | 5 to < 10% |
| Extremely unlikely | 1 to < 5% |
| Almost impossible | 0 to < 1% |
For the assessments of the prohibitions of movements of animals, germinal products and products and material of animal or non‐animal origin (ToR 4.1), assessments of the probability that the activity can lead to transmission of the disease agent 2 ranging from 1% to 100% were considered as confirmatory answers (i.e. a prohibition of the activity is considered necessary), while assessments of the probability that the activity can lead to transmission of the disease agent ranging between 0% and 1% were considered as negative answers (i.e. a prohibition of the activity is considered unnecessary). It should be noted that, as defined in the Problem formulation section, this assessment considered the known biological course of the infection to identify all routes through which an infected animal can potentially shed the agent and the organs of an infected animal that can contain the disease agent. This assessment for prohibition has a binary outcome because if any possibility exists that the activity can lead to spread (here identified as a probability ≥ 1%), a prohibition should be put in place. Transmission of the disease agent is understood as the infection of another animal with the disease agent by direct or indirect means. The transmission risks assessed in this document refer only to transmission to animals; the assessment of zoonotic diseases did not consider public health risks and the assessment of vector‐borne diseases did not consider the presence of nor the attractiveness of the commodities for vectors.
For the assessments of the risk‐mitigation treatments (ToR 4.2 a and b), which included not only risk‐mitigation treatments already listed in Annexes VII and VIII (ToR 4.2. a), but also other treatments that had been identified through the ELS (ToR 4.2. b), assessments of the probability that the treatment can inactivate the disease agent 3 ranging from 90% to 100% were considered as confirmatory answers (i.e. the treatment is considered effective), while assessments of the probability that the treatment can inactivate the disease agent ranging between 0% and < 33% were considered as negative answers (i.e. the treatment is considered ineffective). Assessments of the probability that the treatment can inactivate the disease agent ranging between 33% and < 90% were considered as inconclusive answers (i.e. insufficient evidence exists to conclude on the effectiveness of the treatment). The assessment of the risk mitigation treatments has three potential outcomes with probability ranges of different widths to account for the variability of the treatments' effect. While each treatment results in a log reduction of the disease agent present, the probability that the treatment can entirely inactivate the disease agent in a particular commodity depends on multiple factors, e.g. the infection status of the animal and the resulting amount of the disease agent initially present in the product or material and how effective the treatment is to reduce the disease agent level to below an infectious dose. For a confirmatory answer regarding the effectiveness of the treatment, greater certainty and clear evidence that the disease agent does not survive the treatment in the product or material under consideration was considered to be key. Therefore, the experts decided to use a narrow probability range (90–100%). A larger degree of uncertainty, i.e. a larger range between the upper and lower bound, was allowed for the probability range of a negative outcome (0 to < 33%), and the large probability range (33 to < 90%) for the inconclusive outcome reflects the variability of the treatment effectiveness.
For the second stage, individual judgements were anonymised, summarised graphically (see Appendix B), and collated in a report that included all judgements for each prohibition/risk‐mitigating treatment along with the reasoning provided by the experts. The report was then shared with the experts, who had the opportunity to revise their judgements in the light of the points raised by others.
In the third stage of the assessment, the medians of the lower bounds and of the upper bounds of the probability ranges provided by each expert in stage 2 were used to calculate a median range for each question‐disease/agent combination (‘group range’). Questions in which the group range overlapped more than one outcome option (prohibitions: confirmative, negative; risk‐mitigating treatments: ineffective, inconclusive, effective) were the focus of a group discussion. During the group discussion, the individual probability ranges and the group range were graphically displayed, and the experts discussed the reasoning for their individual assessments and were given the opportunity to change or confirm their individual answers. The reasoning points related to a possible change of answer were recorded. The final outcome is the median of the updated individual probability ranges for a given question‐disease/agent combination.
2.3. Synthesis
The results of the evidence assessment and uncertainty analysis were summarised in a tabular format for each assessment question, showing the outcome for each hazard‐question combination. For ToR 4.2, the outcome tables also include assessments of additional risk‐mitigation treatments identified through the ELS (ToR 4.2 b).
Based on these results, a concluding section was drafted for each Annex of the DR, highlighting the reasons for divergence of the expert assessments from the prohibitions and treatments currently listed in the Annexes VI, VII and VIII, followed by recommendations. Specifically, for the risk mitigation treatments which were not considered effective or for which a conclusion could not be reached, recommendations were made. In addition, where the experts considered that further treatment processes for additional products would be required to ensure safe trade, recommendations were made.
2.4. Uncertainty
Sources of uncertainty related to the assessment of the prohibitions and risk mitigation treatments were identified by the experts in their rationales for each answer and were reflected in the width of the subjective probability ranges supplied in their individual assessments, which in turn was reflected in the group range provided as the final outcome of the assessment.
3. Assessment
3.1. ToR 4.1: Assessment of prohibitions of activities concerning animals and products related to Category A diseases in Annex VI of the DR
This assessment concerns prohibitions in relation to activities, including movements, concerning animals, products and other material within, from or to the protection zone. Previous opinions related to each Category A disease and the recommendations for sizes of the restricted zones have covered the risk associated with spread pathways from the infected establishment to other animals in the zones. The majority of risk by aerosol or fomite transmission is considered to be contained within the zone, but this opinion considers whether spread through the movement of live animals can occur outside these zones. This assessment does not take account of the possibility of non‐compliance.
3.1.1. Assessment of prohibitions regarding movements of animals
To assess the effectiveness of prohibitions regarding movements of animals set out in Annex VI of the DR (Table 2) to control the spread of the Category A diseases (ToR 4.1), the experts estimated the risk that an infected animal of the listed species (i.e. kept animals, and, where relevant, game animals) can transmit the disease agent as a result of the activity listed in Annex VI. To this end, experts considered, based on the known biological course of the infection and the scientific evidence identified through the ELS, if and through which routes an infected animal sheds the disease agent, and which organ systems of an infected animal can contain the disease agent. It was assumed that no specific mitigating measures are applied to the animals prior to their movement.
Table 2.
Prohibitions of movements of animals listed in Annex VI of the DR
| Prohibited movements of animals |
|---|
| Movements of kept animals of listed species from establishments in the restricted zone |
| Movements of kept animals of listed species to establishments in the restricted zone |
| Restocking of game animals of listed species |
| Fairs, markets, shows and other gatherings of kept animals of listed species including collection and dispersion of those species |
| Itinerant natural service of kept animals of listed species |
For all diseases where the primary transmission pathway is direct transmission (foot and mouth disease (FMD), rinderpest (RP), sheep and goat pox (SPGP), CBPP, CCPP, PPR, classical swine fever (CSF), African swine fever (ASF), highly pathogenic avian influenza (HPAI) and Newcastle disease (ND), the experts considered it 99–100% likely (almost certain) that the causative agent can be spread during the listed activities (Figure 1). This is based on scientific evidence showing that infected animals can transmit these disease agents directly and also indirectly.
Figure 1.
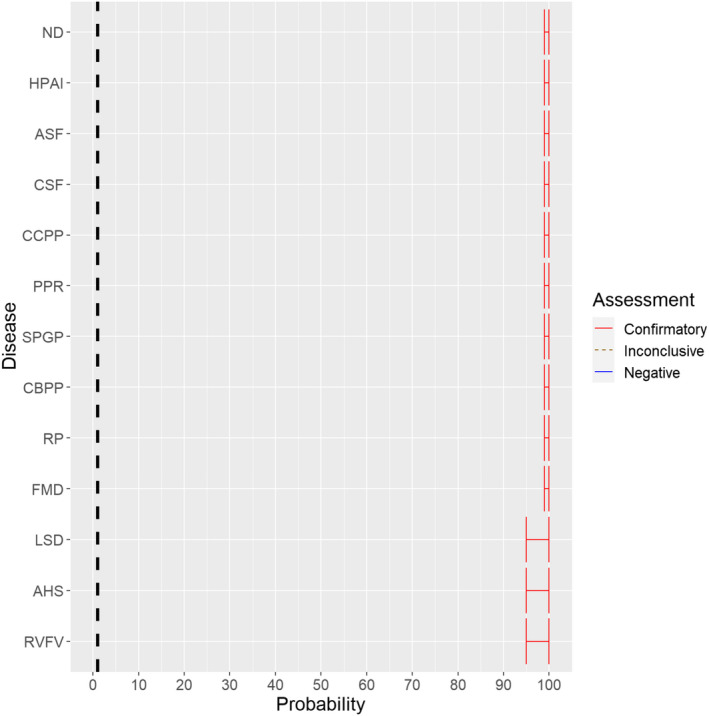
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of the causative agents of the Category A diseases as a result of the movements of animals listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
For the diseases which are only transmitted by vectors (AHS) or where the primary transmission pathway is through biological or mechanical vectors (RVF, lumpy skin disease (LSD)), the experts considered it 95–100% likely (extremely likely to almost certain) that these diseases can be transmitted as a result of the activities. The uncertainty expressed by the experts is linked to the variability of the risk of vector‐borne spread in different geographical areas, seasons and temperature conditions.
As no game animal species in Europe are susceptible to AHS, the experts considered the prohibition of restocking of game animals in Europe as not applicable for this disease.
3.1.2. Assessment of prohibitions regarding movements of germinal products
To assess the effectiveness of prohibitions regarding movements of germinal products set out in Annex VI of the DR (Table 3) to control the spread of the Category A diseases (ToR 4.1), experts estimated the possibility that the germinal products collected or derived from an infected animal of the listed species (i.e. kept animals, and, where relevant, game animals) in the restricted zone can contain the disease agent. To this end, experts considered, based on the known biological course of the infection, reproductive biotechnology methods usually applied, and the scientific evidence identified through the ELS, if semen or oocytes of an infected animal can contain the disease agent, and if in vivo and in vitro embryos derived or produced with semen or oocytes from infected animals can contain the disease agent. It was assumed that no specific mitigating measures have been applied to the germinal products or their donors and that the germinal products have undergone only routine treatments after their collection, e.g. addition of antimicrobials.
Table 3.
Prohibitions of activities regarding germinal products listed in Annex VI of the Commission Delegated Regulation (EU) 2020/687
| Prohibited activities regarding germinal products |
|---|
| Movements of semen, oocytes and embryos obtained from kept animals of listed species from establishments in the restricted zone |
| Collection of semen, oocytes, and embryos from kept animals of listed species |
| Itinerant artificial insemination of kept animals of listed species |
| Movements of hatching eggs from establishments in the restricted zone |
The experts made the following general considerations regarding the contamination risk associated with the germinal products listed in Annex VI:
Disease agents contained in semen cannot be completely removed; therefore, semen has been considered a high‐risk product. The collection of semen from the epididymis of dead males could potentially collect any disease agent present in adjacent tissues.
Oocytes with an intact zona pellucida may still have attachments to cells where disease agents could adhere and replicate. The removal of these cells demands not only washes but further treatment and handling in vitro. In vitro procedures seem to lower the susceptibility of embryos to infections. The collection of oocytes from the ovary is likely to also collect any disease agent present in adjacent tissues, in particular during the viraemic or bacteraemic period. Therefore, an oocyte is considered to be riskier than an in vivo derived embryo.
If contaminated semen or/and oocytes are used for in vitro embryo production, the risk of the resulting embryo to be contaminated has been considered higher than in vivo derived embryos. Moreover, in vitro embryo production processes might increase the susceptibility to contamination.
The risk of in vivo derived embryos to be contaminated has been considered lower than of in vitro produced embryos, as any disease agent contained in the contaminated semen has less probability to reach the oocyte due to the passage through the female genital tract. In addition, during collection of the embryo of an infected female, potentially contaminated cells/liquids present in the genital tract can be collected.
If a contaminated embryo dies due to the presence of the disease agent, the material collected from the animal could be contaminated as well.
Hatching eggs can contain the disease agent either inside or on the shell. If an egg containing an infected embryo breaks before hatching is completed, it is possible that the infectious disease agent is released into the environment.
While the handling of the germinal products certainly poses the possibility to contaminate the products in an infected area, this option has not been part of the assessment done.
3.1.2.1. Foot and mouth disease virus
The experts considered it possible with a limited degree of uncertainty that semen of infected animals can contain infectious foot and mouth disease virus (FMDV) as scientific studies have demonstrated the presence of FMDV in semen of infected bulls.
Regarding in vivo derived embryos and oocytes, the possibility of containing FMDV is influenced by the donor species. For cattle, scientific evidence indicates that the risk might be low (McVicar et al., 1986; Mebus and Singh, 1991; Stringfellow and Givens, 2000). As all susceptible species were considered together, the range of uncertainty is large, and the assessment is inconclusive (Figure 2).
Figure 2.
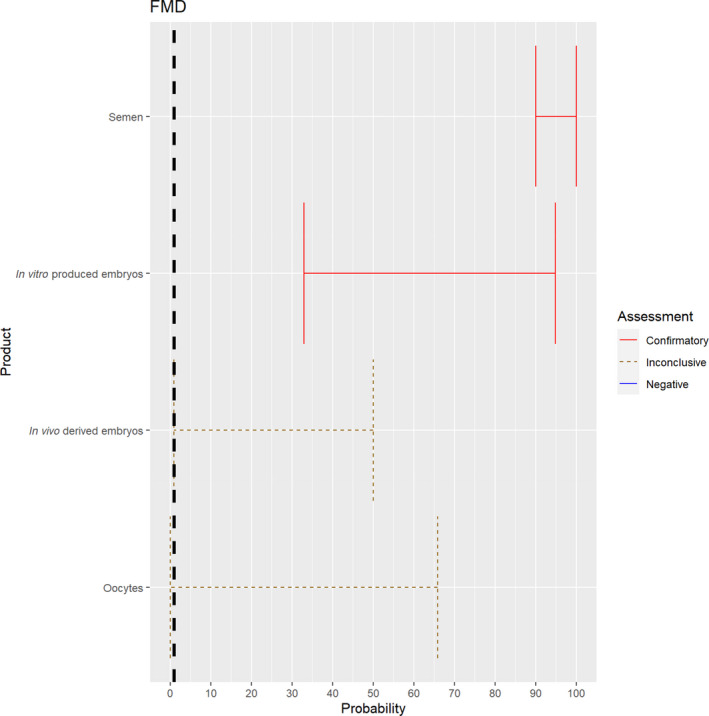
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of presence of FMDV in the germinal products subjected to activities listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.2.2. Rinderpest virus
The experts considered it possible that in addition to semen, oocytes as well as embryos obtained from rinderpest virus (RPV)‐infected animals can contain the infectious pathogen, but as only two publications were identified for oocytes and embryos, which reported contradictory findings (Mebus, 1987; Bielanski, 2014), the assessment of these germinal products is inconclusive and the uncertainty is large (Figure 3).
Figure 3.
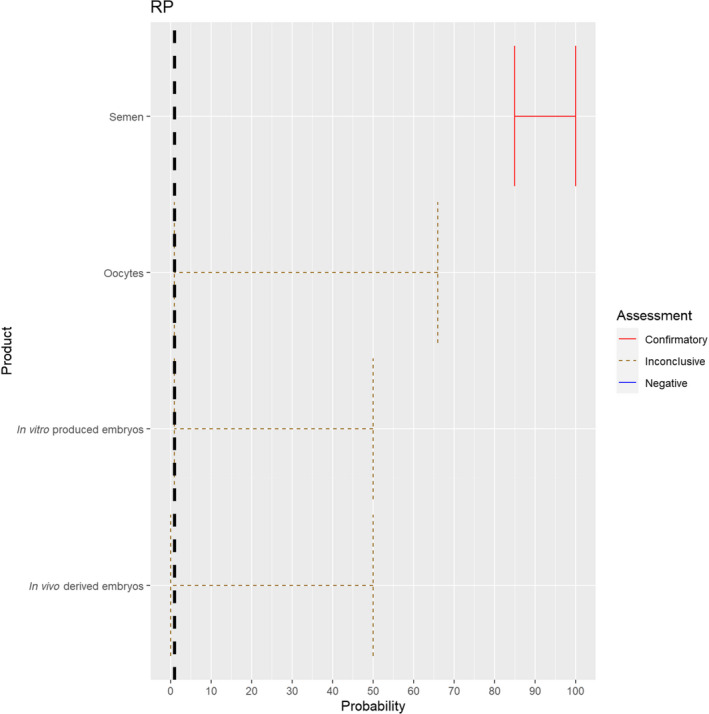
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of presence of RPV in the germinal products subjected to activities listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.2.3. Peste des petits ruminants virus
The experts considered it possible that semen, oocytes as well as embryos obtained from peste des petits ruminants virus (PPRV)‐infected animals can contain the infectious pathogen. Due to a lack of specific scientific evidence, the uncertainty is large (Figure 4).
Figure 4.
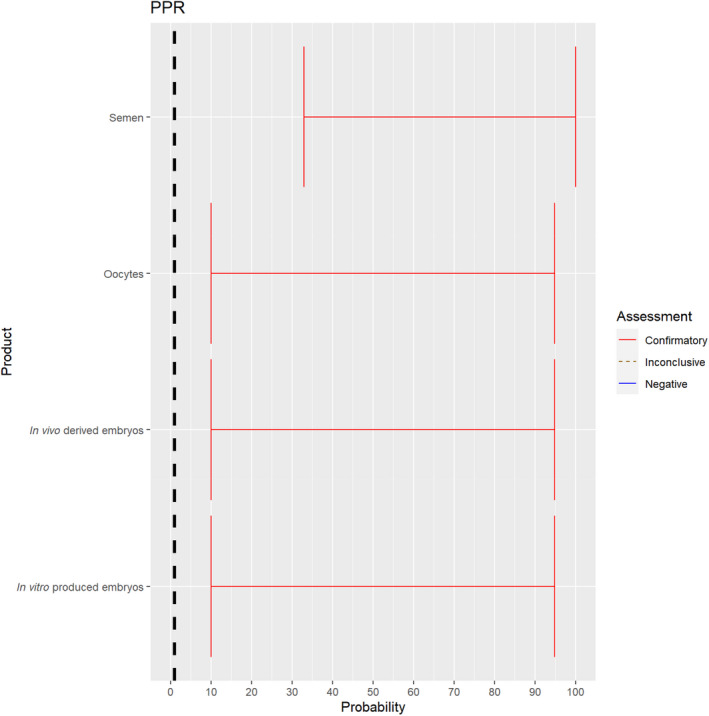
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of presence of PPRV in the germinal products subjected to activities listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.2.4. Rift Valley fever virus
The experts considered it possible that semen, oocytes as well as embryos obtained from Rift Valley fever virus (RVFV)‐infected animals can contain the infectious pathogen, but due to a lack of specific scientific evidence regarding oocytes and embryos, the uncertainty regarding these germinal products is large (Figure 5).
Figure 5.
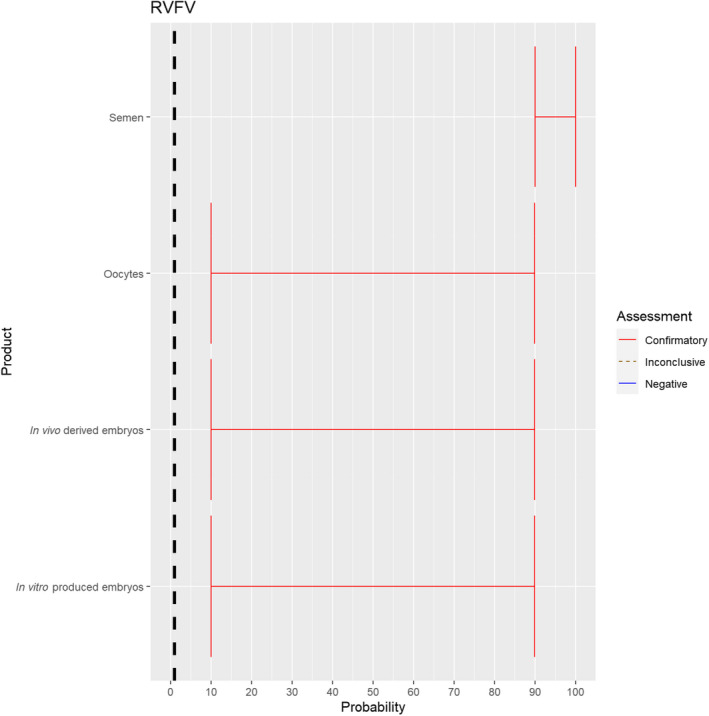
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of presence of RVFV in the germinal products subjected to activities listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.2.5. Lumpy skin disease virus
The experts considered it possible that semen, oocytes as well as embryos obtained from lumpy skin disease virus (LSDV)‐infected animals can contain the infectious pathogen, but due to a lack of specific scientific evidence regarding oocytes and embryos, the uncertainty regarding these germinal products is large (Figure 6). It has been shown that cows inseminated with LSDV‐spiked semen can become infected and the embryos harvested from these cows can become externally contaminated (Annandale et al., 2014).
Figure 6.
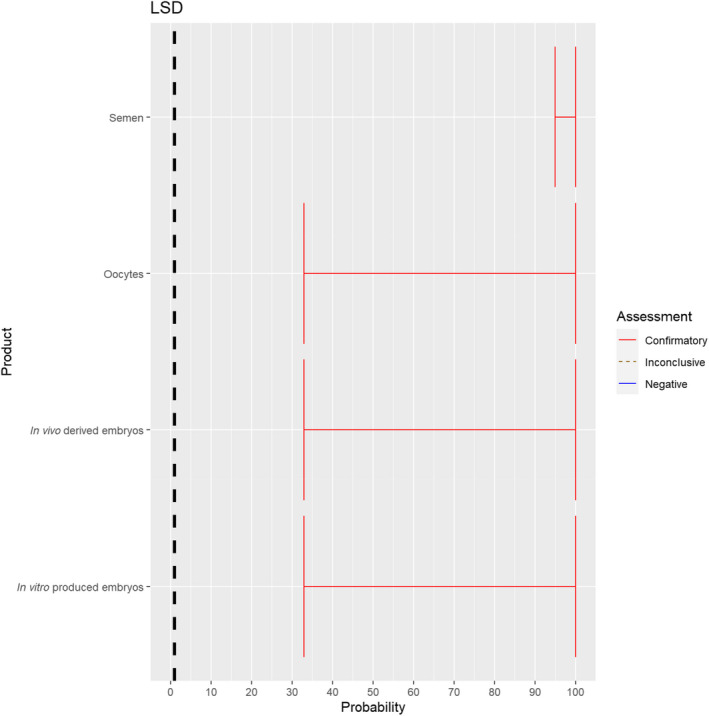
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of presence of LSDV in the germinal products subjected to activities listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.2.6. Sheep and goat pox virus
The experts considered it possible that semen, oocytes as well as embryos obtained from sheep and goat pox virus (SPGPV)‐infected animals can contain the infectious pathogen, but due to a lack of specific scientific evidence, the uncertainty is large (Figure 7).
Figure 7.
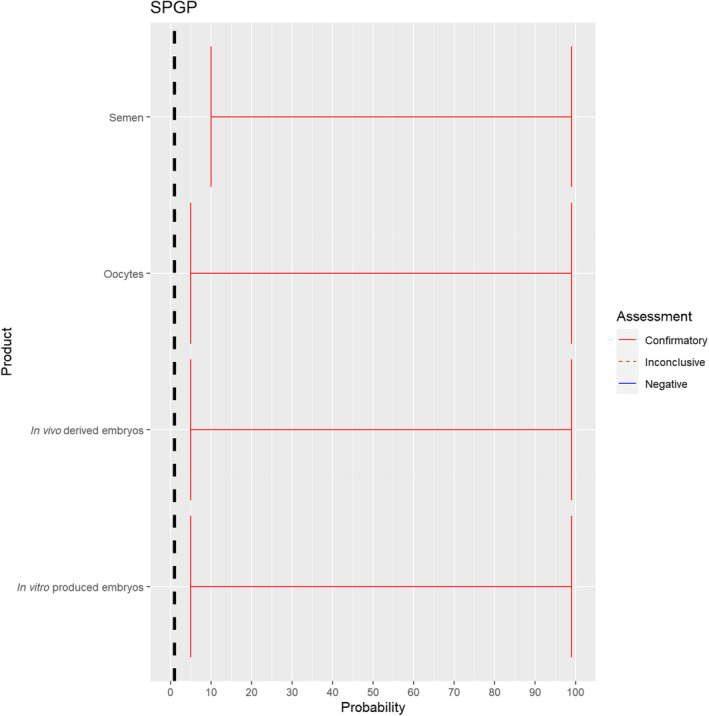
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of presence of SPGPV in the germinal products subjected to activities listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.2.7. Contagious bovine pleuropneumonia
The experts considered it possible that semen, oocytes as well as embryos obtained from animals infected with Mycoplasma mycoides subspecies mycoides can contain the infectious pathogen, but due to a lack of specific scientific evidence, the uncertainty is considerable (Figure 8).
Figure 8.
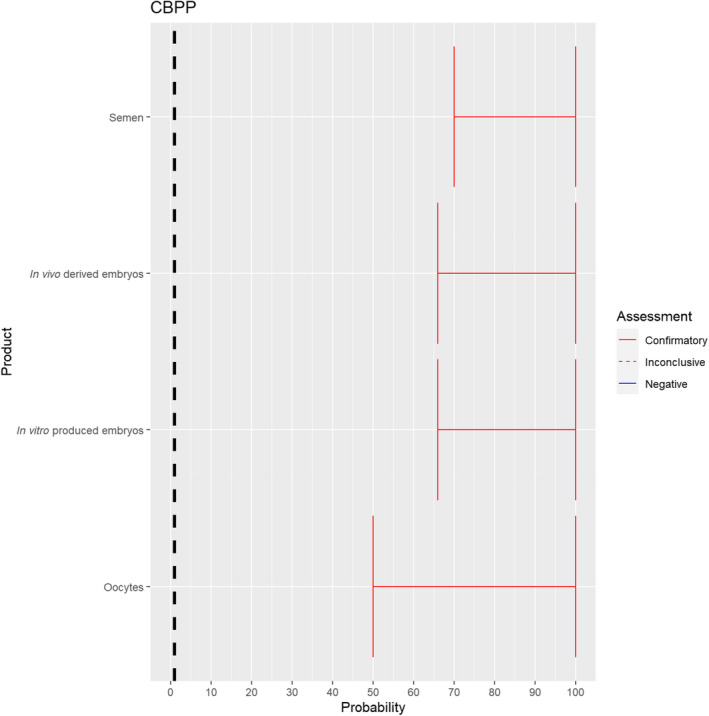
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of presence of Mycoplasma mycoides subspecies mycoides in the germinal products subjected to activities listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.2.8. Contagious caprine pleuropneumonia
The experts considered it possible that semen, oocytes as well as embryos obtained from animals infected with Mycoplasma capricolum subsp. capripneumoniae can contain the infectious pathogen, but due to a lack of specific scientific evidence, the uncertainty is considerable (Figure 9).
Figure 9.
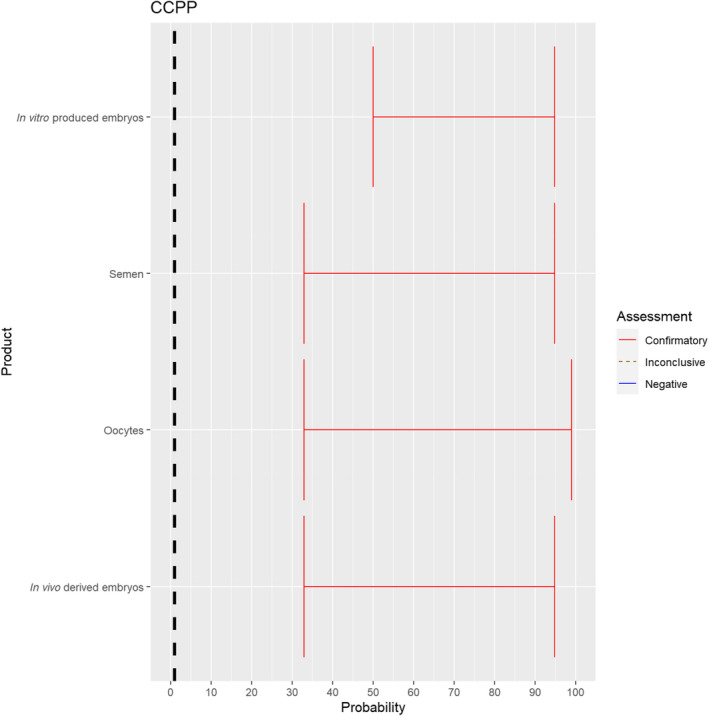
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of presence of Mycoplasma capricolum subsp. capripneumoniae in the germinal products subjected to activities listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.2.9. Classical swine fever virus
The experts considered it possible that semen, oocytes as well as embryos obtained from classical swine fever virus (CSFV)‐infected animals can contain the infectious pathogen, but due to a lack of specific scientific evidence for oocytes and embryos, the uncertainty regarding these germinal products is large. For semen, sufficient scientific evidence existed to derive an assessment with lower uncertainty (Figure 10).
Figure 10.
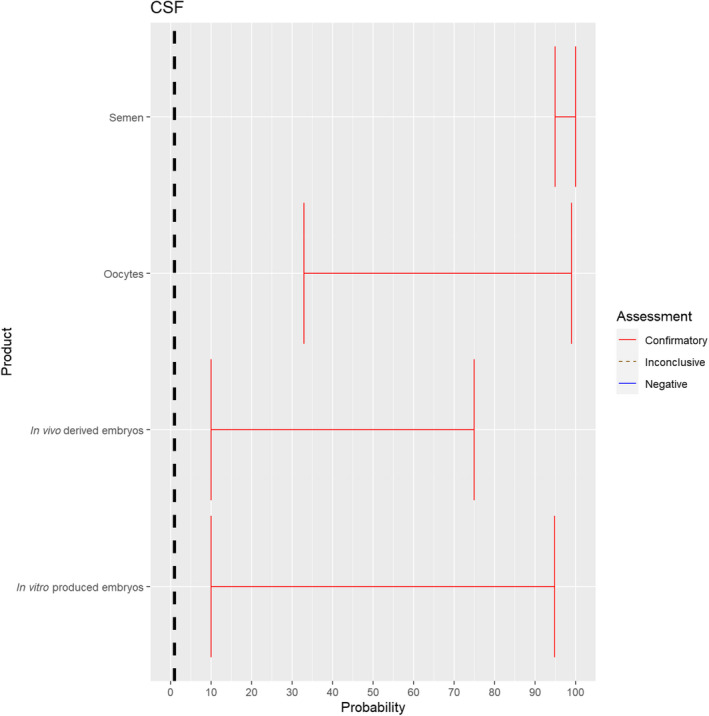
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of presence of CSFV in the germinal products subjected to activities listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.2.10. African swine fever virus
Recent scientific studies have shown the presence of infectious African swine fever virus (ASFV) in the semen of infected boars and the ability to transmit the disease both through natural mating and through artificial insemination to sows (personal communication, Sandra Blome). The experts considered it possible that semen, oocytes as well as embryos obtained from ASFV‐infected animals can contain the infectious pathogen, but due to a lack of specific scientific evidence for oocytes, the uncertainty regarding this germinal product is large (Figure 11).
Figure 11.
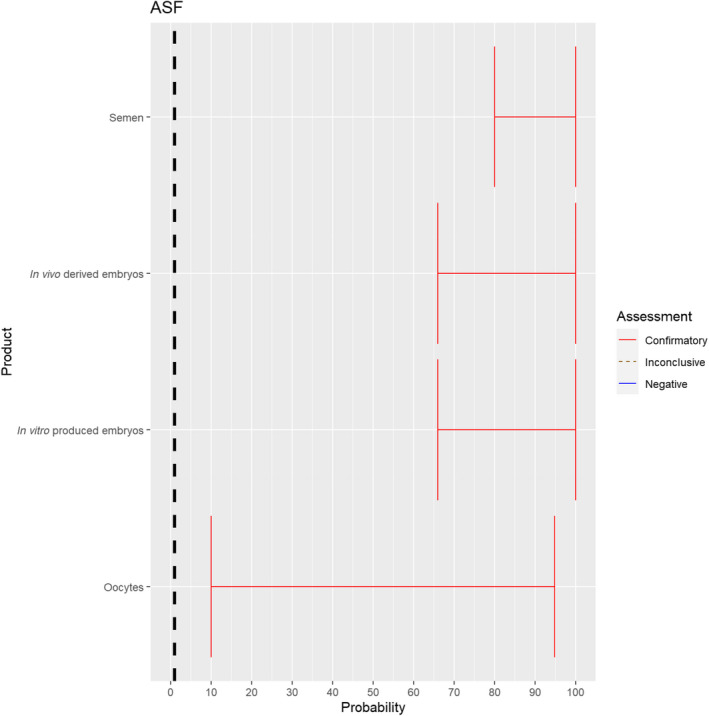
Median of the lower and upper bounds of the subjective probability (in %) ranges expressed by individual experts in the group discussion regarding the possibility of presence of ASFV in the germinal products subjected to activities listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.2.11. African horse sickness virus
The experts considered it possible that semen and oocytes obtained from African horse sickness virus (AHSV)‐infected animals can contain the infectious pathogen, but due to a limited amount of specific scientific evidence for oocytes, the uncertainty regarding this germinal product is large (Figure 12). For embryos, scientific evidence is lacking, therefore the assessment is inconclusive and the uncertainty large.
Figure 12.
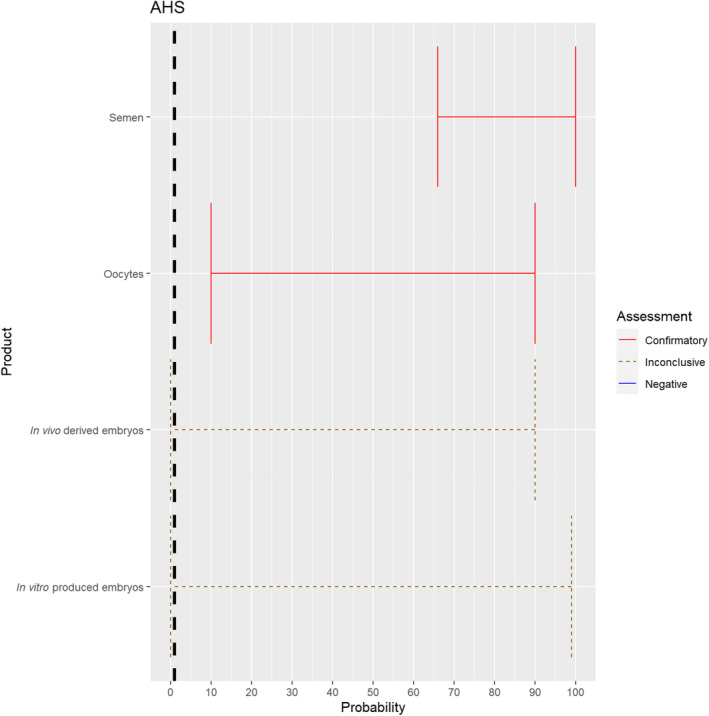
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of presence of AHSV in the germinal products subjected to activities listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.2.12. Highly pathogenic avian influenza virus
The experts considered it possible that semen and hatching eggs obtained from highly pathogenic avian influenza virus (HPAIV)‐infected poultry can contain the infectious pathogen, but due to a limited amount of specific scientific evidence for hatching eggs, some uncertainty regarding this germinal product remains (Figure 13).
Figure 13.
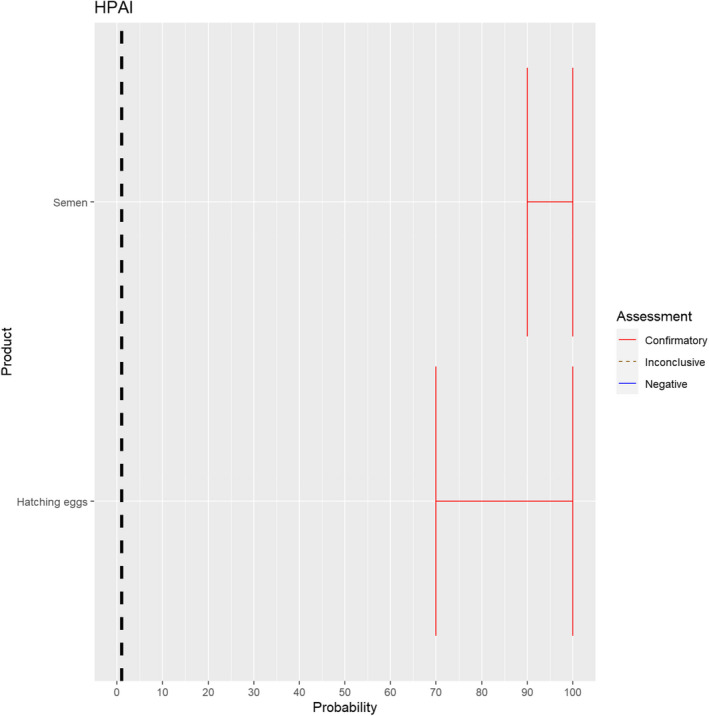
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of presence of HPAIV in the germinal products subjected to activities listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.2.13. Newcastle disease virus
The experts considered it possible that semen and hatching eggs obtained from Newcastle disease virus (NDV)‐infected poultry can contain the infectious pathogen, but due to a limited amount of specific scientific evidence for hatching eggs, some uncertainty regarding this germinal product remains (Figure 14).
Figure 14.
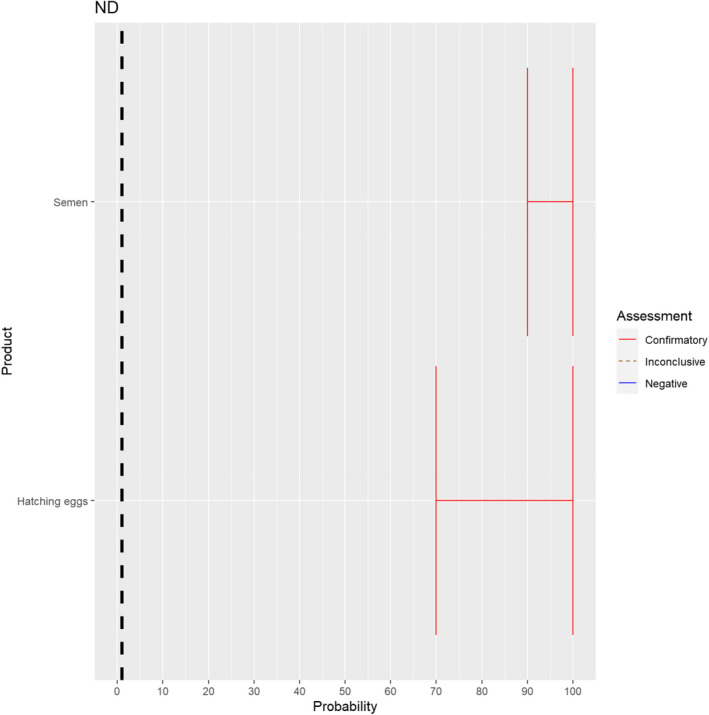
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of presence of NDV in the germinal products subjected to activities listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.3. Assessment of prohibitions regarding movements of animal products, animal by‐products and movements of feed of plant origin and straw
To assess the effectiveness of prohibitions regarding movements of animal products, animal by‐products and movements of feed of plant origin and straw set out in Annex VI of the DR (Table 4) to control the spread of the Category A diseases (ToR 4.1), the experts estimated the possibility that a product can transmit the disease agent as a result of its movement in Annex VI. To this end, the experts considered, based on the known biological course of the infection and the scientific evidence identified through the ELS, the possibility that the infectious disease agent can be present in the original material used to produce the product, the possibility that the product can contain the agent at the end of the production process and the possibility that exposure to this product can lead to infection of a susceptible animal. It was assumed that products have not undergone any specific risk‐mitigating treatment before or after their production.
Table 4.
Assessed activities regarding products and their short names used in figures and text
| Prohibitions of activities concerning animal products, animal by‐products and feed of plant origin and straw related to Category A diseases | Short name used in figures and text |
|---|---|
| Movements of fresh meat excluding offal from kept and wild animals of listed species from slaughterhouses or game handling establishments in the restricted zone | Fresh meat |
| Movements of offal from kept and wild animals of listed species from slaughterhouses or game handling establishments in the restricted zone | Offal |
| Movements of meat products obtained from fresh meat of listed species from establishments in the restricted zone | Meat products |
| Movements of raw milk and colostrum obtained from kept animals of listed species from establishments in the restricted zone | Raw milk |
| Movements of dairy products and colostrum‐based products from establishments in the restricted zone | Dairy products |
| Movements of eggs for human consumption from establishments in the restricted zone | Eggs |
| Movements of animal by‐products from kept animals of listed species from establishments in the restricted zone, except entire bodies or parts of dead animals: manure, including litter and used bedding | Manure |
| Movements of animal by‐products from kept animals of listed species from establishments in the restricted zone, except entire bodies or parts of dead animals: hides, skins, wool, bristles and feathers | Hides, feathers, etc. |
| Movements of animal by‐products from kept animals of listed species from establishments in the restricted zone, except entire bodies or parts of dead animals: animal by‐products other than manure, including litter and used bedding, and other than hides, skins, wool, bristles and feathers | By‐products |
| Movements of feed material of plant origin and straw obtained in the protection zone | Feed |
3.1.3.1. Foot and mouth disease virus
For all animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, the experts considered it possible that their movement can result in spread of FMDV (Figure 15).
Figure 15.
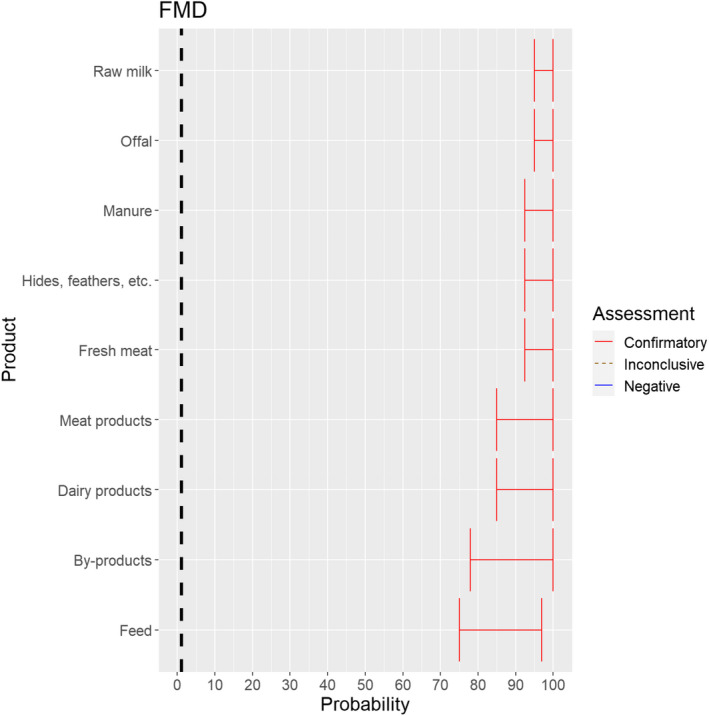
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of FMDV as a result of the movements of animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.3.2. Rinderpest virus
The experts considered it possible that RPV can be spread as a result of movements of the animal products, animal by‐products and feed of plant origin and straw listed in Annex VI (Figure 16).
Figure 16.
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of RPV as a result of the movements of animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.3.3. Peste des petits ruminants virus
The experts considered it possible that PPRV can be spread as a result of movements of the animal products, animal by‐products and feed of plant origin and straw listed in Annex VI (Figure 17).
Figure 17.
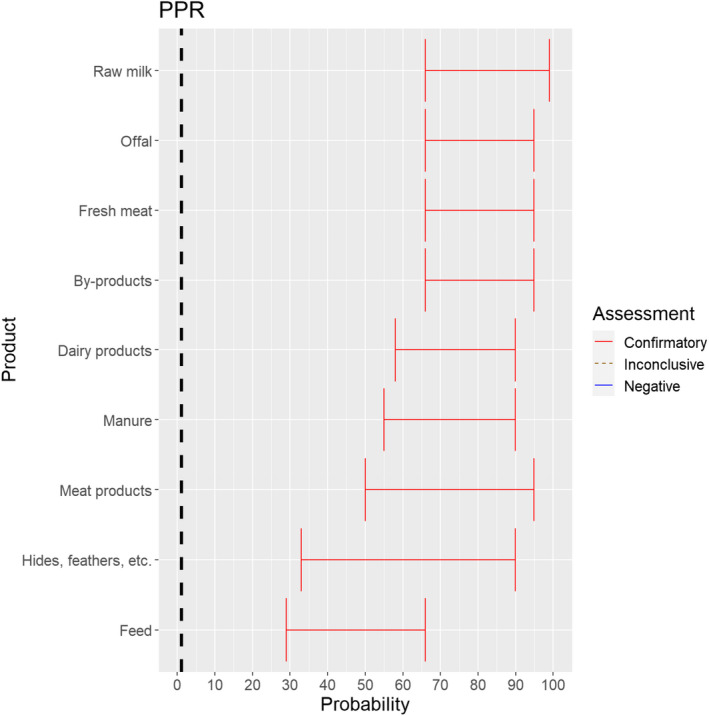
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of PPRV as a result of the movements of animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.3.4. Rift Valley fever virus
The experts considered it possible that RVFV can be spread as a result of movements of the animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, with the exception of meat products and feed, for which the assessment was inconclusive (Figure 18). For meat products of RVFV‐infected animals, it was considered possible that the meat contains infectious virus, but as RVF is inactivated by pH below 6.2 it would be destroyed in meat that has matured (Ellin, 2010; Meegan, 1979). Therefore, it was considered unlikely with a large degree of uncertainty that the movement of meat products leads to RVF spread. Due to the presence of RVFV in vaginal discharges and aborted foetuses and placenta of infected dams, it is possible that the virus may contaminate hides. As no specific scientific references showing the absence of infectious RVFV on hides were identified, the assessment was confirmatory with a low level of probability and large uncertainty.
Figure 18.
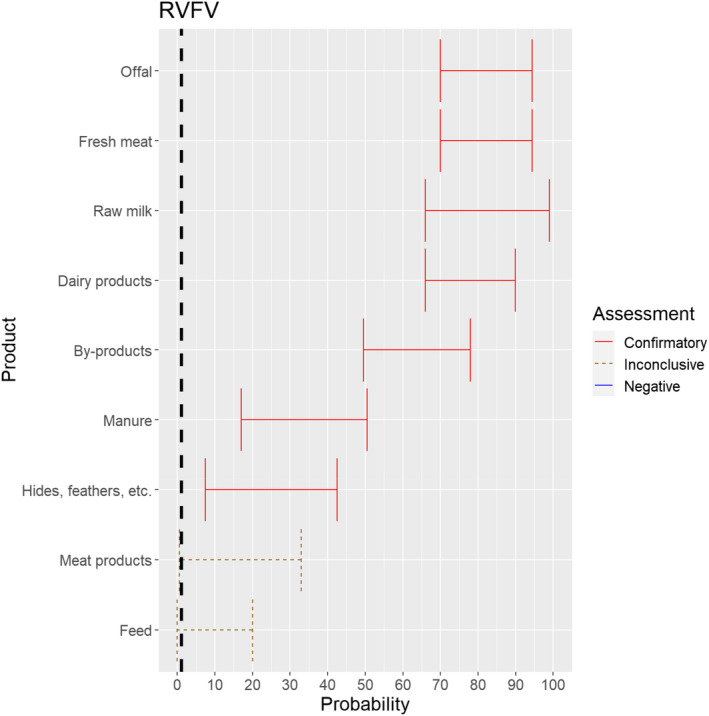
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of RVFV as a result of the movements of animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
Movement of feed, which might be contaminated with RVFV through excretions or abortion material of infected animals, was not considered a likely pathway of transmission, but due to a lack of scientific evidence, the possibility was not excluded.
3.1.3.5. Lumpy skin disease virus
The experts considered it possible that LSDV can be spread as a result of movements of the animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, with the exception of meat products, for which the assessment was inconclusive (Figure 19).
Figure 19.
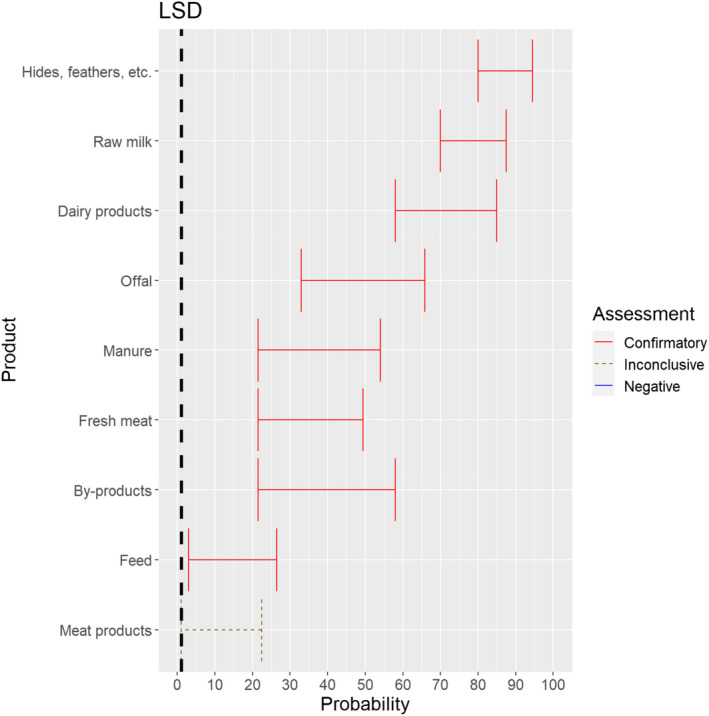
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of LSDV as a result of the movements of animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
The experts considered it possible that meat or lymph nodes of LSDV infected animals can contain infectious virus (Kononov et al., 2019) and that meat products have a lower probability to contain infectious LSDV because of the production methods, which most likely negatively affect virus stability. As LSD is mostly vector borne, it was considered very unlikely that its presence in meat products leads to transmission to animals, but no scientific evidence ruling this out was identified.
3.1.3.6. Sheep and goat pox virus
The experts considered it possible that SPGPV can be spread as a result of movements of the animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, with the exception of fresh meat and meat products, for which the assessment was inconclusive (Figure 20). Infectious particles have been shown to be present in saliva, nasal and conjunctival secretions and virus is also abundant in skin lesions and scabs and can be detected in milk, urine, faeces and semen (CFSPH, 2017).
Figure 20.
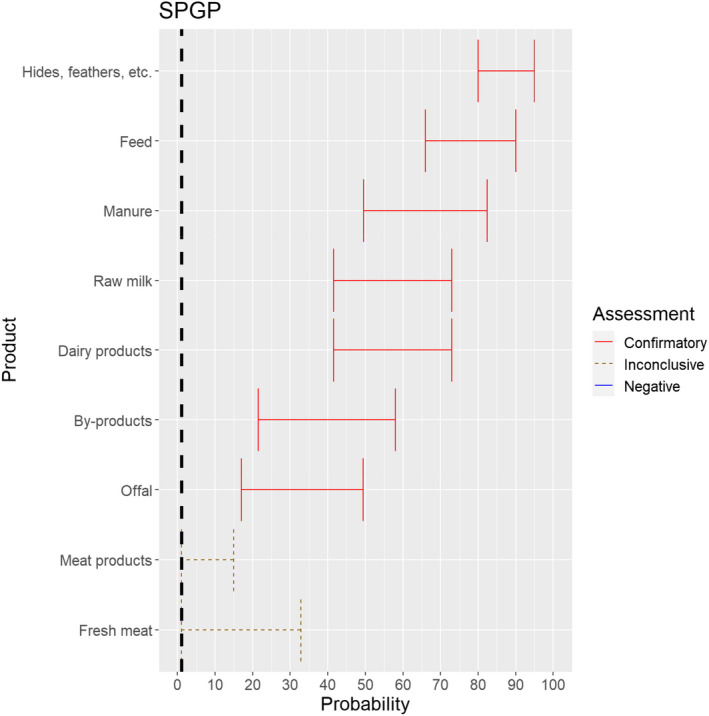
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of SPGPV as a result of the movements of animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
The experts considered it possible that the meat of SPGPV infected animals can contain infectious virus and could not rule out that its presence can lead to transmission to other animals through the movement of fresh meat or meat products due to a lack of scientific evidence.
3.1.3.7. Contagious bovine pleuropneumonia
The experts considered it possible that Mycoplasma mycoides subspecies mycoides can be spread as a result of movements of offal, by‐products, manure and hides. For raw milk, dairy products, fresh meat, meat products and feed, the assessment was inconclusive (Figure 21).
Figure 21.
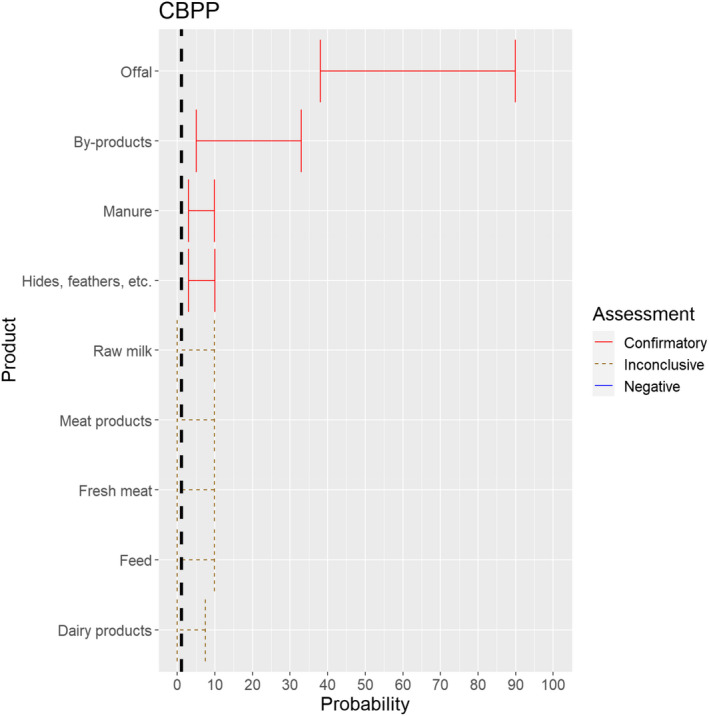
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of Mycoplasma mycoides subspecies mycoides as a result of the movements of animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
The experts considered it not likely that the disease agent is present in fresh meat and meat products of animals infected with Mycoplasma mycoides subspecies mycoides, but the scientific evidence identified did not allow to rule out the possibility of spread through these activities. The same applied to raw milk and dairy products. While feed might be contaminated by secretions of infected animals, the experts considered it not likely that the movement of contaminated feed can lead to spread of the disease. Yet, due to the lack of scientific evidence identified, the possibility could not be ruled out.
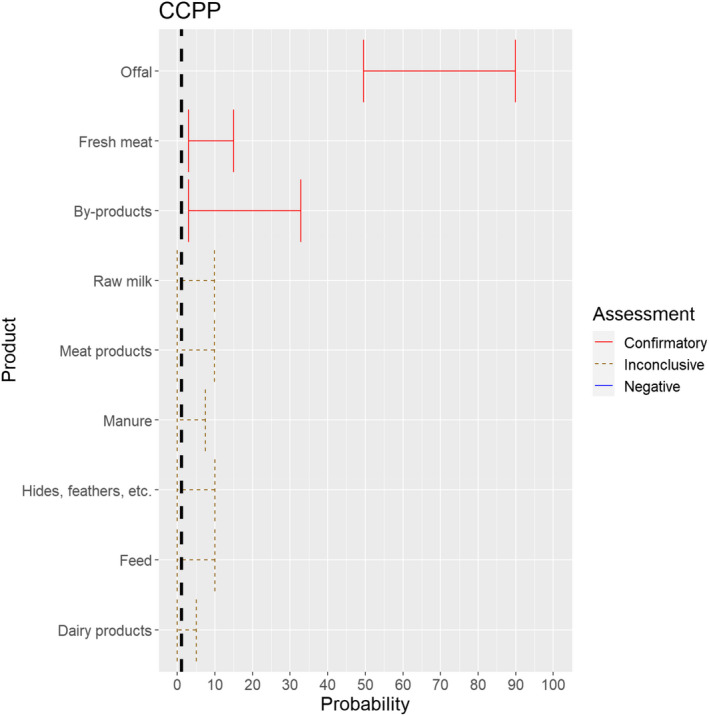
3.1.3.8. Contagious caprine pleuropneumonia
The experts considered it possible that Mycoplasma capricolum subspecies capripneumoniae can be spread as a result of movements of offal, fresh meat and by‐products. For raw milk, dairy products, meat products, hides, manure and feed, the assessments were inconclusive (Figure 22). The experts considered it not likely that the movement of the latter products can lead to spread of the disease, as indirect transmission of CCPP has not been described. However, due to the scarcity of scientific evidence identified, the possibility could not be ruled out.
Figure 22.
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of Mycoplasma capricolum subspecies capripneumoniae as a result of the movements of animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.3.9. Classical swine fever virus
The experts considered it possible that CSFV can be spread as a result of movements of the animal products, animal by‐products and feed of plant origin and straw listed in Annex VI (Figure 23).
Figure 23.
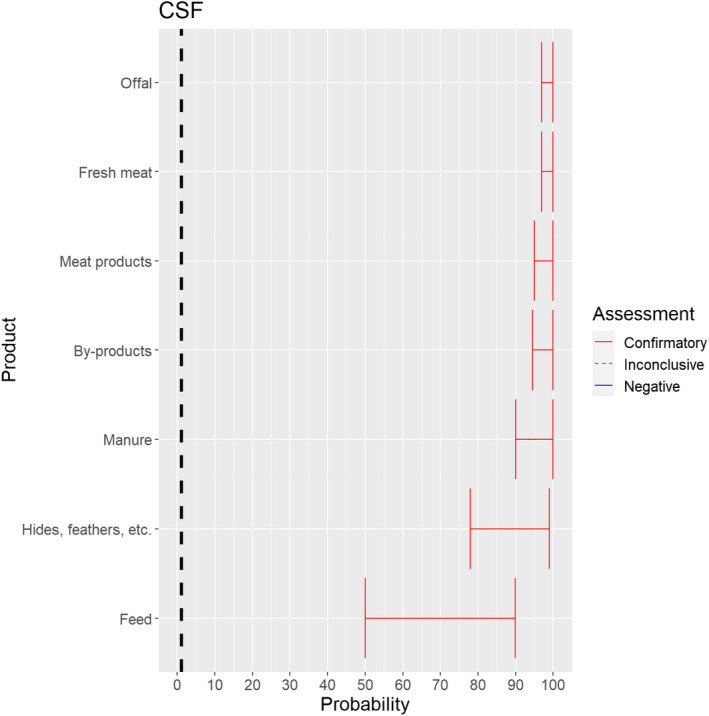
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of CSFV as a result of the movements of animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.3.10. African swine fever virus
The experts considered it possible that ASFV can be spread as a result of movements of the animal products, animal by‐products and feed of plant origin and straw listed in Annex VI (Figure 24).
Figure 24.
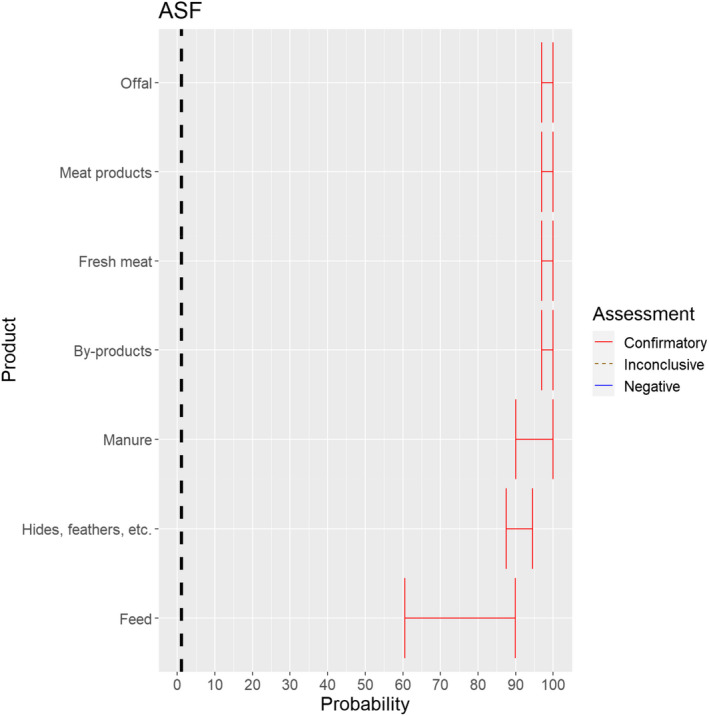
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of ASFV as a result of the movements of animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.3.11. African horse sickness virus
The experts considered it not possible that AHSV can be spread as a result of movements of hides of infected horses or of manure and feed that have been in contact with infected horses, as the virus is not present in any secretions or the skin of infected animals, and the assessment did not consider the presence of nor the attractiveness of the commodities for insect vectors. For offal, fresh meat and meat products, raw milk and dairy products as well as by‐products, the assessment was inconclusive, because the possibility that exposure to this product can lead to an infection of a horse was considered to be extremely low due to the exclusive transmission of the virus through vectors but could not be ruled out completely due to the lack of scientific evidence identified (Figure 25).
Figure 25.
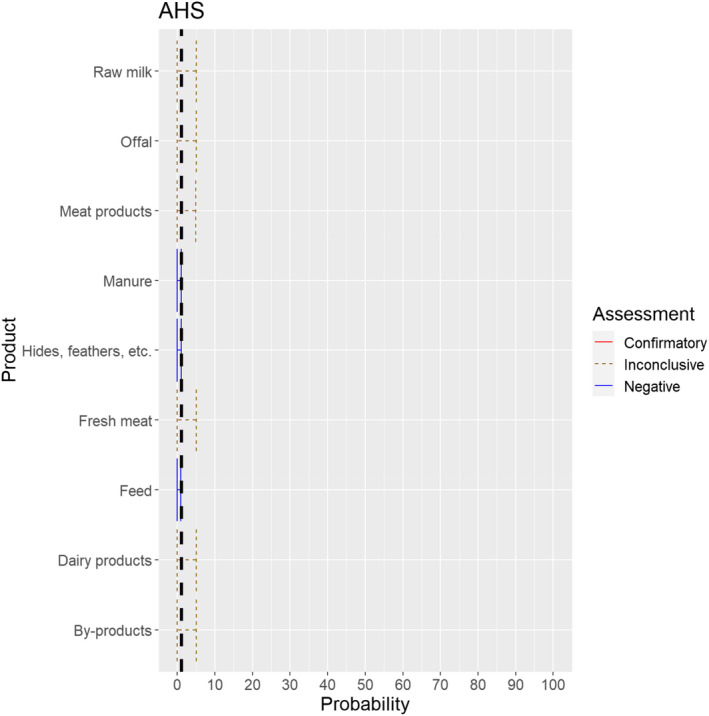
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of AHSV as a result of the movements of animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.3.12. Highly pathogenic avian influenza virus
The experts considered it possible that HPAIV can be spread as a result of movements of the animal products, animal by‐products and feed of plant origin and straw listed in Annex VI (Figure 26).
Figure 26.
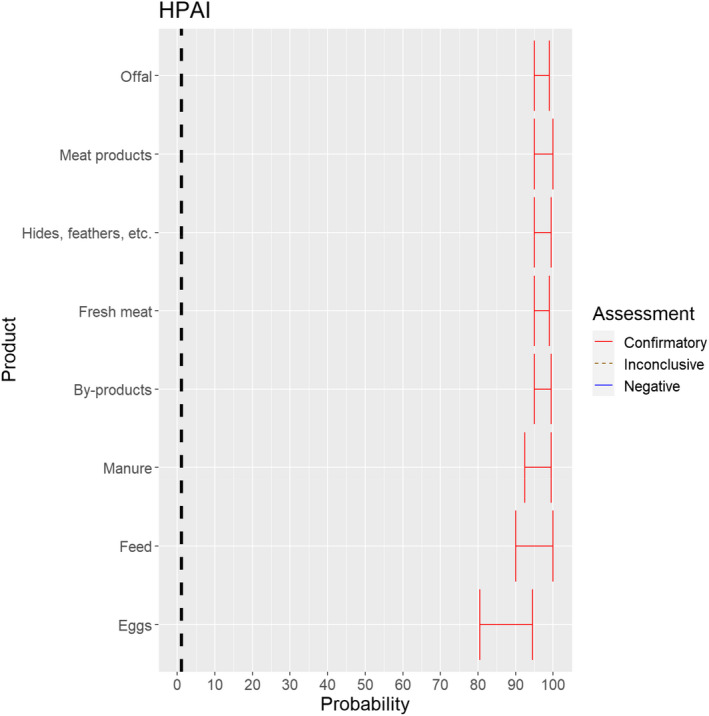
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of HPAIV as a result of the movements of animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.1.3.13. Newcastle disease virus
The experts considered it possible that NDV can be spread as a result of movements of the animal products, animal by‐products and feed of plant origin and straw listed in Annex VI (Figure 27).
Figure 27.
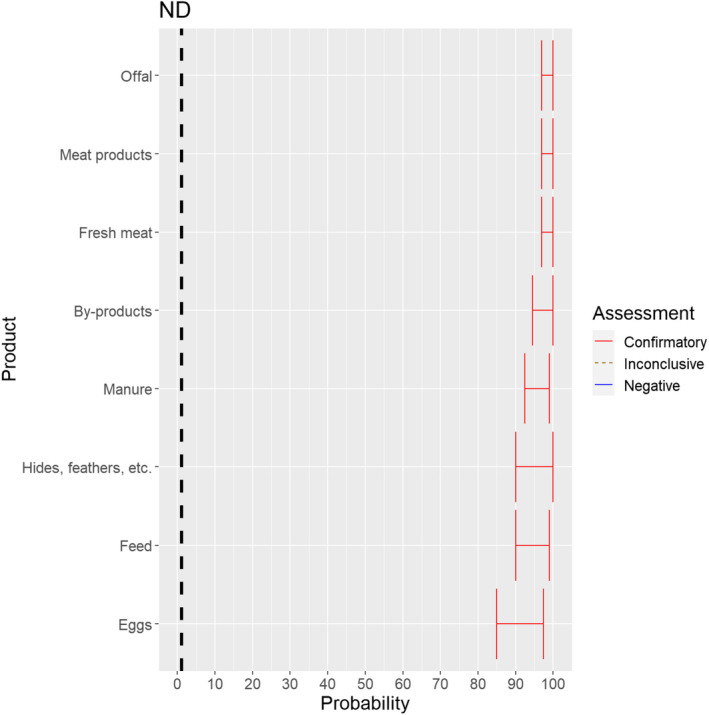
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the possibility of spread of NDV as a result of the movements of animal products, animal by‐products and feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (the vertical line represents the cut‐off between confirmatory answers (> 1%) and negative answers (0–1%))
3.2. ToR 4.2 (a) and (b): Assessment of the effectiveness of risk‐mitigating treatments to control the presence of Category A disease agents in products of animal origin and other relevant materials listed in Annexes VII and VIII of the DR and additional risk‐mitigating treatments identified through the ELS
The assessment of the effectiveness of risk‐mitigating treatments to control the presence of Category A disease agents in products of animal origin and other relevant materials included 51 treatments listed in Annex VII and 2 treatments listed in Annex VIII. The treatment for meat ‘Heat treatment to achieve desiccation to maximum values of Aw of 0.93 and pH of 6’ and the treatments for casings ‘Salting with sodium chloride (NaCl) minimum 30 days’, ‘Bleaching’ and ‘Drying’ listed in Annex VII have not been assessed as they are not prescribed for any of the listed agents/species. In addition, 25 treatments for products of animal origin currently not listed in Annexes VII, which have been identified though the ELS were assessed. The ELS did not identify additional risk mitigation treatments for products of non‐animal origin. Tables 5 and 6 list the assessed risk mitigation treatments and their short names used in the text and the respective figures describing the assessment results.
Table 5.
Assessed risk mitigation treatments for products of animal origin and their short names used in figures and text
| Product | ASSESSED RISK‐MITIGATING TREATMENTS FOR PRODUCTS OF ANIMAL ORIGIN listed in Annex VII | Short name used in figures and text |
|---|---|---|
| Meat | Heat treatment in a hermetically sealed container, to achieve a minimum F0 value of 3 | Meat_1 |
| Heat treatment to achieve a core temperature of 80°C | Meat_2 | |
| Heat treatment to achieve a core temperature of 70°C | Meat_3 | |
| Heat treatment (to meat previously de‐boned and defatted) to achieve a core temperature of 70°C for a minimum of 30 min | Meat_4 | |
| In a hermetically sealed container, applying 60°C for a minimum of 4 h | Meat_5 | |
| Core temperature of 73.9 °C for a minimum of 0.51 s | Meat_6 | |
| Core temperature of 70.0°C for a minimum of 3.5 s | Meat_7 | |
| Core temperature of 65.0°C for a minimum of 42 s | Meat_8 | |
| Core temperature of 60.0°C for a minimum of 507 s | Meat_9 | |
| Heat treatment to achieve a core temperature of 65°C for a period of time to achieve a minimum pasteurisation value of 40 | Meat_11 | |
| Natural fermentation and maturation for bone‐in meat: minimum 9 months, to achieve maximum values of Aw of 0.93 and pH of 6 | Meat_12 | |
| Natural fermentation and maturation for de‐boned meat: minimum 9 months, to achieve maximum values of Aw of 0.93 and pH of 6 | Meat_13 | |
| Natural fermentation for loins: minimum 140 days to achieve maximum values of Aw of 0.93 and pH of 6 | Meat_14 | |
| Natural fermentation for hams: minimum 190 days to achieve maximum values of Aw of 0.93 and pH of 6 | Meat_15 | |
| Drying after salting Italian style bone‐in hams: minimum 313 days | Meat_16 | |
| Drying after salting Iberian hams: minimum 252 days | Meat_17 | |
| Drying after salting Iberian shoulders: minimum 140 days | Meat_18 | |
| Drying after salting Iberian loins: minimum 126 days | Meat_19 | |
| Drying after salting Serrano hams: minimum 140 days | Meat_20 | |
| Maturation of carcasses at a minimum temperature of 2°C for a minimum of 24 h following slaughter | Meat_21 | |
| Removal of offal | Meat_22 | |
| Casings | Salting with sodium chloride (NaCl) either dry or as saturated brine (Aw < 0.80), for a continuous period of 30 days or longer at an ambient temperature of 20°C or above | Casings_1 |
| Salting with phosphate supplemented salt 86.5% NaCl, 10.7% Na2HPO4 and 2.8% Na3PO4 either dry or as saturated brine (Aw < 0.80) for a continuous period of 30 days or longer at an ambient temperature of 20°C or above | Casings_2 | |
| Milk | Heat treatment (sterilisation process) to achieve a minimum F0 value of 3 | Milk_1 |
| Heat treatment UHT (ultra‐high temperature): Minimum 132°C for a minimum of 1 s | Milk_2 | |
| Heat treatment UHT (ultra‐high temperature): Minimum 135°C for a suitable holding time | Milk_3 | |
| Heat treatment HTST (High‐temperature short‐time) pasteurisation if milk pH is lower than 7, minimum 72°C for a minimum of 15 s | Milk_4 | |
| Heat treatment HTST (High‐temperature short‐time) pasteurisation if milk pH is 7 or higher, minimum 72°C for a minimum of 15 s, applied twice | Milk_5 | |
| Heat treatment HTST (High‐temperature short‐time) pasteurisation combined with a physical treatment to achieve pH value below 6 for a minimum of 1 h or heat treatment HTST to achieve a minimum of 72°C, combined with desiccation | Milk_6 | |
| Pasteurisation consisting in a single heat treatment with an effect at least equivalent to that achieved by applying 72°C for 15 s | Milk_7 | |
| Eggs | Whole egg: 60°C ‐ 188 s | Egg_1 |
| Whole egg: completely cooked | Egg_2 | |
| Whole egg blends: 60°C ‐ 188 s | Egg_3 | |
| Whole egg blends: 61.1°C ‐ 94 s | Egg_4 | |
| Whole egg blends: completely cooked | Egg_5 | |
| Liquid egg white: 55.6°C ‐ 870 s | Egg_6 | |
| Liquid egg white: 56.7°C ‐ 232 s | Egg_7 | |
| Plain or pure egg yolk: 60°C ‐ 288 s | Egg_8 | |
| 10% salted yolk: 62.2°C ‐ 138 s | Egg_9 | |
| Dried egg white: 67°C ‐ 20 h | Egg_10 | |
| Dried egg white: 54.4°C ‐ 50.4 h | Egg_11 | |
| Dried egg white: 51.7°C ‐ 73.2 h | Egg_12 | |
| Whole egg: 55°C ‐ 2521 s | Egg_13 | |
| Whole egg: 57°C ‐ 1596 s | Egg_14 | |
| Whole egg: 59°C ‐ 674 s | Egg_15 | |
| Whole egg: completely cooked | Egg_16 | |
| Liquid egg white: 55°C ‐ 2278 s | Egg_17 | |
| Liquid egg white: 57°C ‐ 986 s | Egg_18 | |
| Liquid egg white: 59°C ‐ 301 s | Egg_19 | |
| 10% salted egg yolk: 55°C ‐ 176 s | Egg_20 | |
| Dried egg white: 57°C ‐ 54.0 h | Egg_21 | |
| Product | ASSESSED RISK‐MITIGATING TREATMENTS FOR PRODUCTS OF ANIMAL ORIGIN identified by the ELS | Short name used in figures and text |
| Casings | Salting with citrate‐supplemented salt 89.2% NaCl, 8.9% trisodium citrate dehydrate and 1.9% citric acid monohydrate (wt/wt/wt), with pH 4.5 for a continuous period of 30 days or longer at an ambient temperature of 20°C or above | Alt_casings1 |
|
Eggs |
Dried egg white: 54.4°C ‐ 21.38 days | Alt_egg1 |
| Liquid whole egg: 64.4°C – 200 s | Alt_egg2 | |
| Fortified egg: 61.1°C – 6.2 min | Alt_egg3 | |
| Fortified egg: 62.2°C – 3.5 min | Alt_egg4 | |
| sugared/salted egg: 62.2°C – 6.2 min | Alt_egg5 | |
| sugared/salted egg: 63.3°C – 3.5 min | Alt_egg6 | |
| plain yolk: 60°C – 6.2 min | Alt_egg7 | |
| plain yolk: 61.1°C – 3.5 min | Alt_egg8 | |
| Meat | Drying after salting Serrano hams: minimum 182 day | Alt_meat1 |
| Heat treatment to achieve a core temperature of 70°C for at least 30 min | Alt_meat2 | |
| Drying after salting Italian style bone‐in hams: minimum 400 days | Alt_meat3 | |
| Drying after salting (Italian style) loins: minimum 137 days | Alt_meat4 | |
| Core temperature of 70°C for a minimum of 5 s | Alt_meat5 | |
| Core temperature of 60°C for a minimum of 60 min | Alt_meat6 | |
| Incubation at 500 MPa at 15°C for a minimum of 15 s | Alt_meat7 | |
| Core temperature of 65.0°C for a minimum of 120 s | Alt_meat8 | |
| Core temperature of 70.0°C for a minimum of 82 s | Alt_meat9 | |
| Core temperature of 74.0°C for a minimum of 40 s | Alt_meat10 | |
| Core temperature of 80.0°C for a minimum of 29 s | Alt_meat11 | |
| Core temperature above 70°C for a minimum of 82 s | Alt_meat12 | |
| Core temperature of 57.8°C for a minimum of 63.3 min | Alt_meat13 | |
| Milk | Pasteurisation consisting in a single heat treatment with an effect at least equivalent to that achieved by applying 72°C for 15 s with additional acidification | Alt_milk1 |
| Heat treatment HTST (High‐temperature short‐time) pasteurisation combined with a physical treatment to achieve pH value below 6 for a minimum of 1 h or High temperature short time pasteurisation to achieve a minimum of 72°C, combined with desiccation | Alt_milk2 | |
| Heat treatment UHT (ultra‐high temperature): Minimum 132°C for a minimum of 1 s, combined with another physical treatment | Alt_milk3 |
Table 6.
Assessed risk mitigation treatments for products of non‐animal origin and their short names used in figures and text
| Product | ASSESSED RISK‐MITIGATING TREATMENTS FOR PRODUCTS OF NON‐ANIMAL ORIGIN listed in Annex VIII | Short name used in figures and text |
|---|---|---|
| Feed materials of plant origin and straw | Heat treatment, minimum temperature of 80°C and for a minimum of 10 min, steam in a closed chamber | Non_anim_prod1 |
| Storage in package or bales under shelter at premises situated not closer than 2 km to the nearest outbreak and releasing from the premises do not take place before at least three months have elapsed following the completion of cleaning and disinfection according to Article 15 | Non_anim_prod2 |
3.2.1. Assessment of the effectiveness of risk‐mitigating treatments for products of animal origin from the restricted zone (listed in Annex VII of the DR and additional treatments identified though the ELS)
For the purpose of this opinion, for the treatments which are not considered effective or for which evidence was inconclusive, further recommendations are given.
3.2.1.1. Foot and mouth disease virus
Twenty‐one treatments for products of animal origin listed in Annex VII and one additional treatment identified by the ELS were assessed for FMDV. Overall, there were 10 treatments that were considered effective, 2 treatments that were considered ineffective and 10 for which there was inconclusive evidence (Figure 28).
Figure 28.
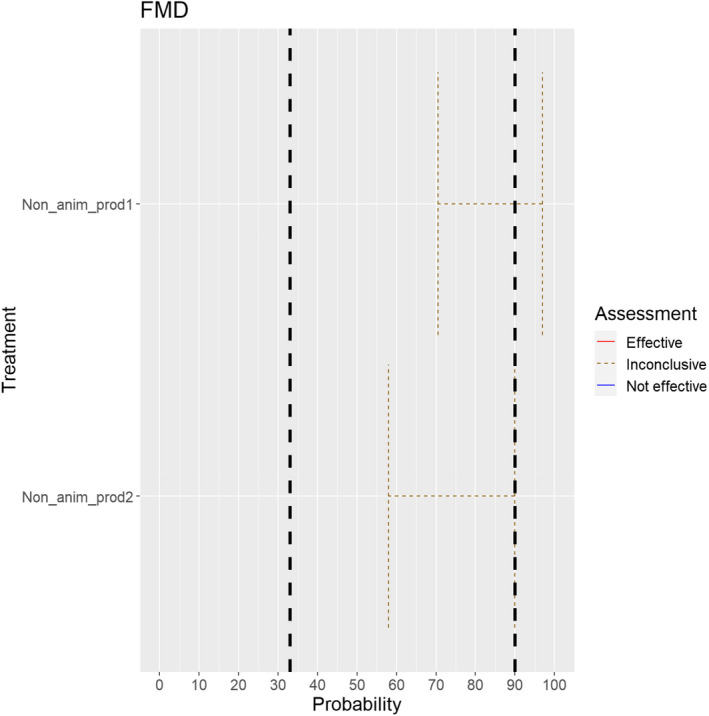
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the effectiveness of treatments for animal products to mitigate the risk for FMDV (the vertical lines represent cut‐offs for categorisation of treatments as not effective (< 33%), inconclusive (33 to < 90% and medians spanning two or more areas) or effective (90–100%))
The ELS results were based on 15 references, which determined experimental effects of the treatments on the FMDV in particular products. There were two publications relating to curing and cooking meat from infected animals, two related to brining casings from infected cattle, nine relating to milk treatment. Therefore, the evidence was incomplete and many of the assessments were based on the general properties of the virus in culture, organs and the environment, or on the current practices prescribed by the WOAH Terrestrial animal health code (WOAH, 2021) or EU legislation.
For FMD, there were no additional products, which were considered to require further treatment processes to ensure safe trade.
The following 10 risk mitigation treatments were considered effective:
-
•
Meat heat treated in a hermetically sealed container to F0 (Treatment Meat_1).
-
•
Meat heat treatment (to meat previously de‐boned and defatted) to achieve a core temperature of 70°C for a minimum of 30 min (Treatment Meat_4).
-
•
Natural fermentation and maturation for de‐boned meat: minimum 9 months, to achieve maximum values of Aw of 0.93 and pH of 6 (Treatment Meat_13).
-
•
Drying after salting Iberian hams: minimum 252 days (Treatment Meat_17).
-
•
Drying after salting Iberian shoulders: minimum 140 days (Treatment Meat_18).
-
•
Drying after salting Iberian loins: minimum 126 days (Treatment Meat_19).
-
•
Salting with sodium chloride (NaCl) either dry or as saturated brine (Aw < 0.80), for a continuous period of 30 days or longer at an ambient temperature of 20°C or above (Treatment Casings_1).
-
•
Salting with phosphate supplemented salt 86.5% NaCl, 10.7% Na2HPO4 and 2.8% Na3PO4 either dry or as saturated brine (Aw < 0.80) for a continuous period of 30 days or longer at an ambient temperature of 20°C or above (Treatment Casings_2).
-
•
Heat treatment (sterilisation process) to achieve a minimum F0 value of 3 (Treatment Milk_1).
-
•
Drying after salting Serrano hams: minimum 182 days (Treatment Alt_Meat1).
The following two treatments were considered not effective:
-
•
Milk heat treatment HTST (High‐temperature short‐time) pasteurisation if milk pH is lower than 7, minimum 72°C for a minimum of 15 s (Treatment Milk_4): Several studies mention that one HTST treatment is not sufficient to completely inactivate FMDV (Blackwell and Hyde, 1976; Dhennin and Labie, 1976, Salwa and Gaber, 2007, Tomasula et al., 2007). Whether the condition of pH lower than 7 will be sufficient to inactivate FMDV is doubtful, as Sonder et al. (1990) showed that neither acidification nor hydrogen peroxide treatment were reliable for the inactivation of FMDV in skimmed milk.
-
•
Milk pasteurisation consisting of a single heat treatment with an effect at least equivalent to that achieved by applying 72°C for 15 s (Treatment Milk_7): Evidence has already been presented that this treatment time is not long enough to completely inactivate FMD virus (Blackwell and Hyde, 1976; Dhennin and Labie, 1976; Salwa and Gaber, 2007; Tomasula et al., 2007).
The following 10 treatments were considered inconclusive:
-
•
Meat heat‐treated to achieve a core of temperature of 80°C (Treatment Meat_2): The experts considered this treatment inconclusive, as the treatment description should also indicate for how long the core temperature of 80°C should be held. Evidence from the ELS only looked at temperatures of 71°C and 75°C, and the time specified for each was 10.66 h and 5.75 h, respectively (Masana et al., 1995).
-
•
Meat heat‐treated to achieve a core of temperature of 70°C (Treatment Meat_3): The experts considered this treatment inconclusive, as the treatment description should also indicate for how long the core temperature of 70°C should be held. The evidence is the same as for Treatment Meat_2, but for this treatment the necessary time period is likely to be > 10.66 h (Masana et al., 1995).
-
•
Meat treated in a hermetically sealed container, applying 60°C for a minimum of 4 h (Treatment Meat_5): While WOAH recommends heat treatment of meat in a hermetically sealed container to reach an internal core temperature of at least 70°C for a minimum of 30 min (WOAH, 2021), no evidence for this specific temperature–time combination has been identified. Therefore, the assessment of this treatment was inconclusive.
-
•
Meat heated to a core temperature of 73.9°C for a minimum of 0.51 s (Treatment Meat_6): The ELS reported that temperatures of 71°C and 75°C at a time of 10.66 h and 5.75 h, respectively, were effective in inactivating the virus (Masana et al., 1995). This evidence at the lower temperature suggests a longer period is required. As no evidence for this specific time–temperature combination was identified, the treatment assessment is inconclusive.
-
•
Natural fermentation and maturation for bone‐in meat: minimum 9 months, to achieve maximum values of Aw of 0.93 and pH of 6 (Treatment Meat_12): The treatment assessment is inconclusive, in particular, as the effect of fermentation on the bone marrow is not known. Therefore, uncertainty regarding the persistence of the virus in the bone marrow exists.
-
•
Drying after salting Serrano hams: minimum 140 days (Treatment Meat_20): The ELS identified only one study on FMDV survival in Serrano ham, which indicated that a minimum of 182 days would be needed for virus inactivation Mebus et al. (1997). Therefore, the evidence for the effectiveness of this treatment was considered inconclusive.
-
•
Milk heat treatment UHT (Ultra‐high temperature): Minimum 132°C for a minimum of 1 s (Treatment Milk_2): The ELS reported FMD virus is inactivated in milk at 65°C for 30 s (Kästli and Moosbrugger, 1968), but only after 55 s at 72°C without acidification (Sellers, 1969). As no study demonstrating the effectiveness of this specific time–temperature combination has been identified, the evidence was considered inconclusive.
-
•
Milk heat treatment UHT (Ultra‐high temperature): Minimum 135°C for a suitable holding time (Treatment Milk_3): The information on this treatment was considered inconclusive because no holding time is specified. The ELS reported that a holding time of 2.5 s at 148°C is required for effective treatment (Walker et al., 1984).
-
•
Milk heat treatment HTST (High‐temperature short‐time) pasteurisation if milk pH is 7 or higher, minimum 72°C for a minimum of 15 s, applied twice (Treatment Milk_5): The ELS reported that 55 s are required for milk heated to 72°C to inactivate the virus, where no acidification is applied (Sellers, 1969). There is no evidence for the effect of the treatment being applied twice for 15 s.
-
•
Milk heat treatment HTST (High‐temperature short‐time) pasteurisation combined with a physical treatment to achieve pH value below 6 for a minimum of 1 h or HTST pasteurisation to achieve a minimum of 72°C, combined with desiccation (Treatment Milk_6): HTST has been shown to achieve a reduction of FMDV in milk, however, acidification is not necessarily effective in inactivating FMDV in milk (Sellers, 1969), and nothing is known about the effect of desiccation on FMDV. Therefore, the assessment of this treatment is inconclusive.
3.2.1.2. Peste des petits ruminants virus
Thirteen treatments listed for PPRV in Annex VII were assessed. Overall, eight treatments were considered effective, and for five treatments there was inconclusive evidence (Figure 29).
Figure 29.
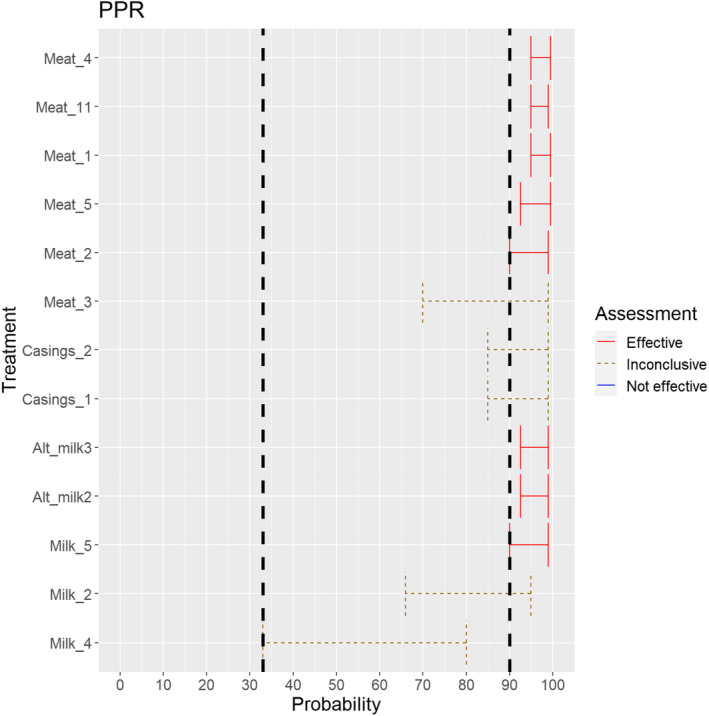
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the effectiveness of treatments for animal products to mitigate the risk for PPR virus (the vertical lines represent cut‐offs for categorisation of treatments as not effective (< 33%), inconclusive (33 to < 90% and medians spanning two or more areas) or effective (90–100%))
The ELS resulted in no references which looked at experimental effects of the treatments on PPR virus in particular products. Therefore, the evidence is incomplete and many of the assessments are based on the general properties of the virus in culture or organs and on the WOAH Terrestrial animal health code (WOAH, 2021) or EU legislation.
For PPRV, there were no additional products for which the experts considered that treatment processes would be required to ensure safe trade.
The following eight treatments were considered effective:
-
•
Meat heat treatment in a hermetically sealed container, to achieve a minimum F0 value of 3 (Treatment Meat_1).
-
•
Meat heat treatment to achieve a core temperature of 80°C (Treatment Meat_2).
-
•
Meat heat treatment (to meat previously de‐boned and defatted) to achieve a core temperature of 70°C for a minimum of 30 min (Treatment Meat_4).
-
•
Meat treated in a hermetically sealed container, applying 60°C for a minimum of 4 h (Treatment Meat_5).
-
•
Heat treatment to achieve a core temperature of 65°C for a period of time to achieve a minimum pasteurisation value of 40 (Treatment Meat_11).
-
•
Heat treatment HTST (High‐temperature short‐time) pasteurisation if milk pH is 7 or higher, minimum 72°C for a minimum of 15 s, applied twice (Treatment Milk_5).
-
•
Heat treatment HTST (High‐temperature short‐time) pasteurisation combined with a physical treatment to achieve pH value below 6 for a minimum of 1 h or HTST to achieve a minimum of 72°C, combined with desiccation (Treatment Alt_milk2).
-
•
Heat treatment UHT (Ultra‐high temperature): Minimum 132°C for a minimum of 1 s, combined with another physical treatment (Treatment Alt_milk3).
For the following five treatments, the evidence was inconclusive:
-
•
Meat heat treatment to achieve a core temperature of 70°C (Treatment Meat_3), the temperature was considered to be potentially effective, however, with no time specification no conclusion could be reached. There are no data on inactivation of PPR virus, but rapid inactivation at temperatures at 70°C and above is expected. The WOAH Code chapter on PPR recommends for the importation that meat should be processed to a minimum internal temperature of 70°C for at least 30 min (WOAH, 2021).
-
•
Casing salting with sodium chloride (NaCl) either dry or as saturated brine (Aw < 0.80), for a continuous period of 30 days or longer at an ambient temperature of 20°C or above (Treatment Casings_1). Although the experts generally concur with a previous EFSA report (EFSA, 2006) that the treatment may be effective, as no experimental data exist, some uncertainty follows this assessment.
-
•
Casing salting with phosphate supplemented salt 86.5% NaCl, 10.7% Na2HPO4 and 2.8% Na3PO4 either dry or as saturated brine (Aw < 0.80) for a continuous period of 30 days or longer at an ambient temperature of 20°C or above (Treatment Casings_2): As for Casings_1, the treatment may be effective, but no experimental data exist, which adds uncertainty to the assessment.
-
•
Milk heat treatment UHT (Ultra‐high temperature): Minimum 132°C for a minimum of 1 s (Treatment Milk_2): No data has been identified by the ELS. Previous assessments by EFSA and WOAH have been based on comparisons to mitigate risks associated with FMDV. However, this treatment was assessed as ineffective for FMDV, and the WOAH Code does not recommend this treatment for milk used for animals. There is a lack of scientific evidence for the effectiveness of this treatment.
-
•
Milk heat treatment HTST (High‐temperature short‐time) pasteurisation if milk pH is lower than 7, minimum 72°C for a minimum of 15 s (Treatment Milk_4): no data for PPRV has been identified. The WOAH Code for PPRV is based on treatments for FMDV, but this treatment is not even considered effective for FMDV. Therefore, this treatment is also considered inconclusive for PPRV.
3.2.1.3. Rift Valley fever virus
Two treatments for products of animal origin listed in Annex VII and one additional treatment for milk identified by the ELS were assessed for RVFV (Figure 30).
Figure 30.
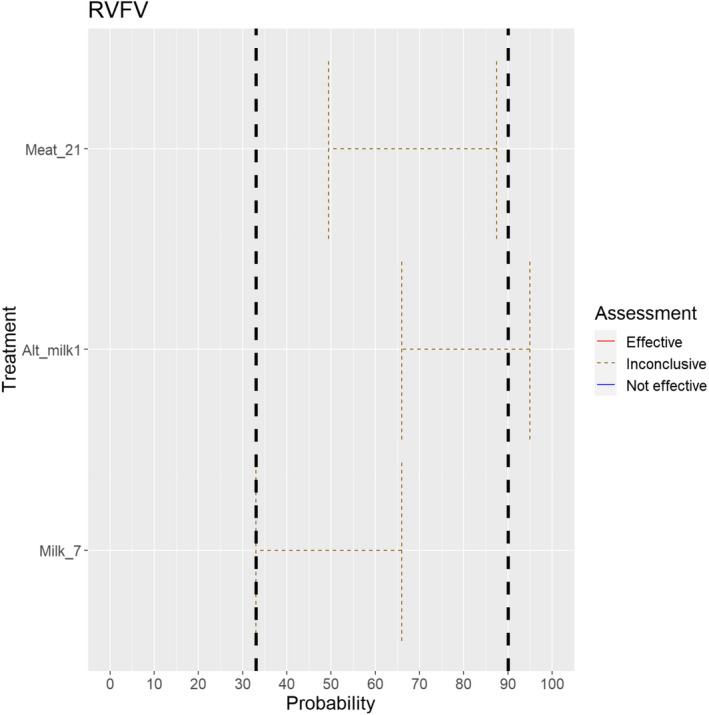
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the effectiveness of treatments for animal products to mitigate the risk for RVF virus (the vertical lines represent cut‐offs for categorisation of treatments as not effective (< 33%), inconclusive (33 to < 90% and medians spanning two or more areas) or effective (90–100%))
For the three treatments, the evidence was inconclusive:
-
•
Maturation of carcasses at a minimum temperature of 2°C for a minimum of 24 h following slaughter (Treatment Meat_temp_21): No data was identified for this specific disease/product combination.
-
•
Milk pasteurisation consisting in a single heat treatment with an effect at least equivalent to that achieved by applying 72°C for 15 s (Treatment Milk_7): No data was identified for this specific disease/product combination. Pasteurisation temperatures would be expected to result in rapid inactivation of the virus. However, EFSA previously concluded that by using heat treatment alone virus infectivity may not be completely removed (EFSA, 2006). Therefore, this treatment might not be totally effective.
-
•
Milk pasteurisation consisting in a single heat treatment with an effect at least equivalent to that achieved by applying 72°C for 15 s with additional acidification (Treatment Alt_milk1): This treatment appears more likely to be effective than regular pasteurisation due to the acidification, but a degree of uncertainty remains due to the lack of specific evidence (the EFSA report suggests this treatment, but no data to support it) (EFSA, 2006).
3.2.1.4. Lumpy skin disease virus
Two treatments for products of animal origin listed in Annex VII and one additional treatment for milk identified by the ELS were assessed for LSDV (Figure 31).
Figure 31.
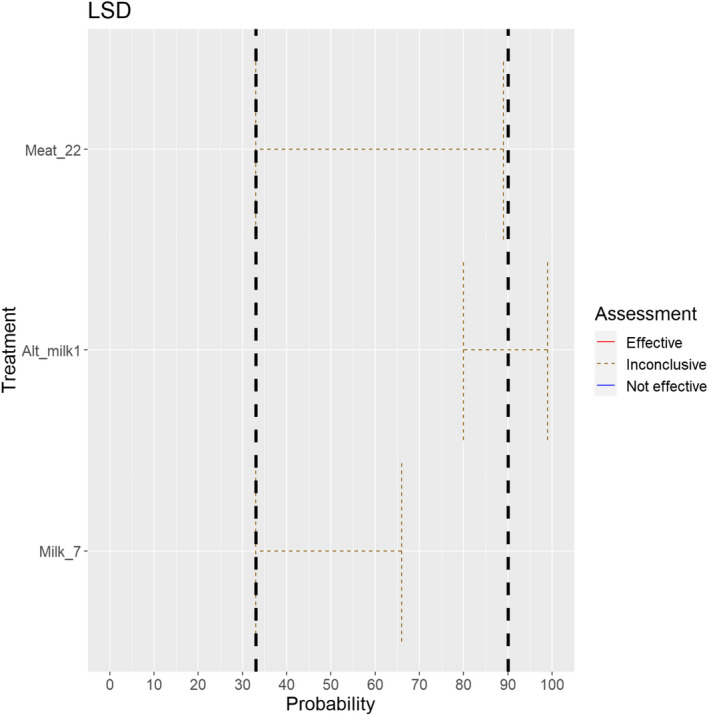
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the effectiveness of treatments for animal products to mitigate the risk for LSD virus (the vertical lines represent cut‐offs for categorisation of treatments as not effective (< 33%), inconclusive (33 to < 90% and medians spanning two or more areas) or effective (90–100%))
For three treatments, the evidence was inconclusive:
-
•
Removal of offal (Treatment Meat_22): LSDV might remain in lymph nodes and testicles of infected animals, whereas deep skeletal meat does not carry live virus and the risk of transmission through this product seems very low (Kononov et al., 2019). If treatment is performed in a proper way, it is likely to be effective. However, uncertainty remains in case of contamination of the carcass or in case pathologic lesions (virus still found in muscles with gross pathology) are not detected and removed at meat inspection, since the virus is resistant to inactivation in such conditions.
-
•
Milk pasteurisation consisting in a single heat treatment with an effect at least equivalent to that achieved by applying 72°C for 15 s (Treatment Milk_7): No data were identified for this specific disease/product combination. Virus is inactivated by heating at 65°C for 30 min. A report mentions that there is evidence that heating at 56°C for 30 min or 60°C for 10 min inactivates virus in milk (ILSI, 2009). However, it is uncertain whether treating milk just 15 s at 72°C is likely to be effective.
-
•
Milk pasteurisation consisting in a single heat treatment with an effect at least equivalent to that achieved by applying 72°C for 15 s with additional acidification (Treatment Alt_milk1): Due to the characteristics of the disease agent, being susceptible to environmental conditions, this treatment is more likely to be effective than regular pasteurisation due to the acidification. However, a degree of uncertainty remains due to the lack of specific evidence.
3.2.1.5. Contagious bovine pleuropneumonia
One treatment listed for CBPP in Annex VII was assessed (Figure 32).
Figure 32.
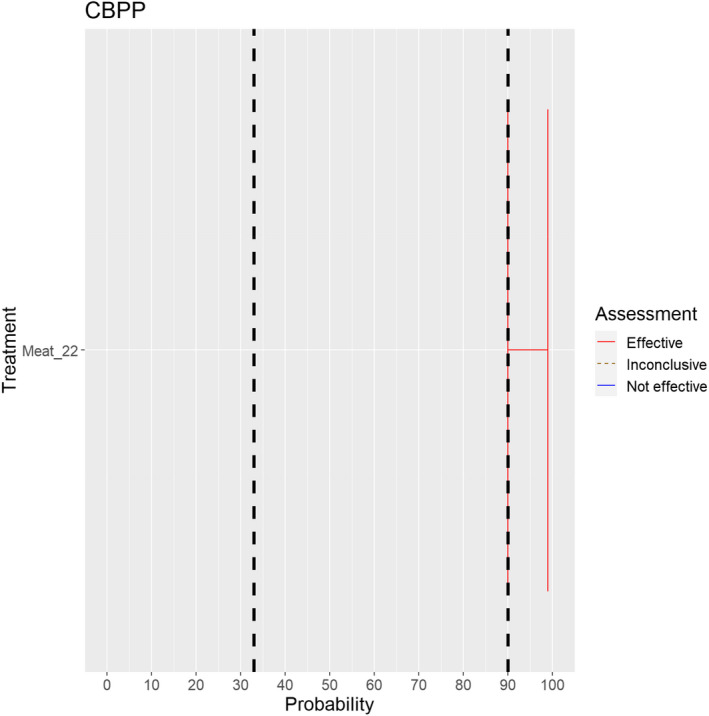
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the effectiveness of treatments for animal products to mitigate the risk for Mycoplasma mycoides subspecies mycoides (the vertical lines represent cut‐offs for categorisation of treatments as not effective (< 33%), inconclusive (33% to < 90% and medians spanning two or more areas) or effective (90–100%))
The ELS resulted in no references studying experimental effects of the treatments regarding CBPP in offal. Therefore, the evidence is incomplete, and the assessment is based on the general properties of the agent and the WOAH Terrestrial animal health code (WOAH, 2021).
Treatment Meat_22 (Removal of offal) has been considered effective by the experts (Figure 32) as the disease agent is not found in deep muscle tissue and as meat is considered a safe commodity by WOAH, even though the scientific basis for this consideration has not been included in the WOAH code.
3.2.1.6. Contagious caprine pleuropneumonia
One treatment listed for CCPP in Annex VII was assessed (Figure 33).
Figure 33.
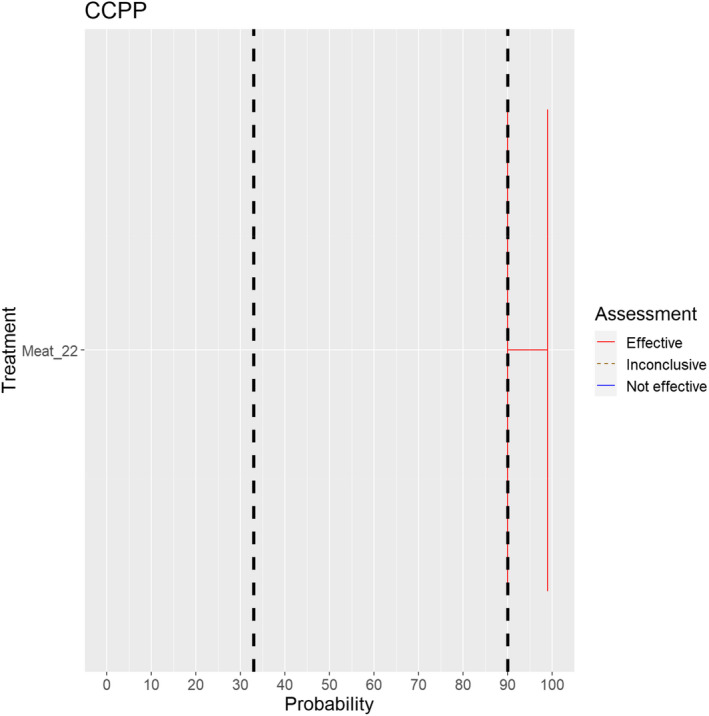
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the effectiveness of treatments for animal products to mitigate the risk for Mycoplasma capricolum subsp. capripneumoniae (the vertical lines represent cut‐offs for categorisation of treatments as not effective (< 33%), inconclusive (33% to < 90% and medians spanning two or more areas) or effective (90–100%))
The ELS resulted in no references, which looked at experimental effects of the treatment on CCPP in offal. Therefore, the evidence is incomplete, and the assessment was based on the general properties of the agent and the WOAH Terrestrial animal health code (WOAH, 2021).
Treatment Meat_22 (Removal of offal) has been considered effective by the experts (Figure 33), as the disease agent is not present in the meat of infected animals (Rapoport and Shimshony, 1997).
3.2.1.7. Classical swine fever virus
A total of 16 treatments listed for CSFV in Annex VII and one new suggested treatment were assessed. Fourteen of these treatments concerned meat products and three concerned casings. Overall, nine treatments were considered effective and for eight treatments there was inconclusive evidence (Figure 34).
Figure 34.
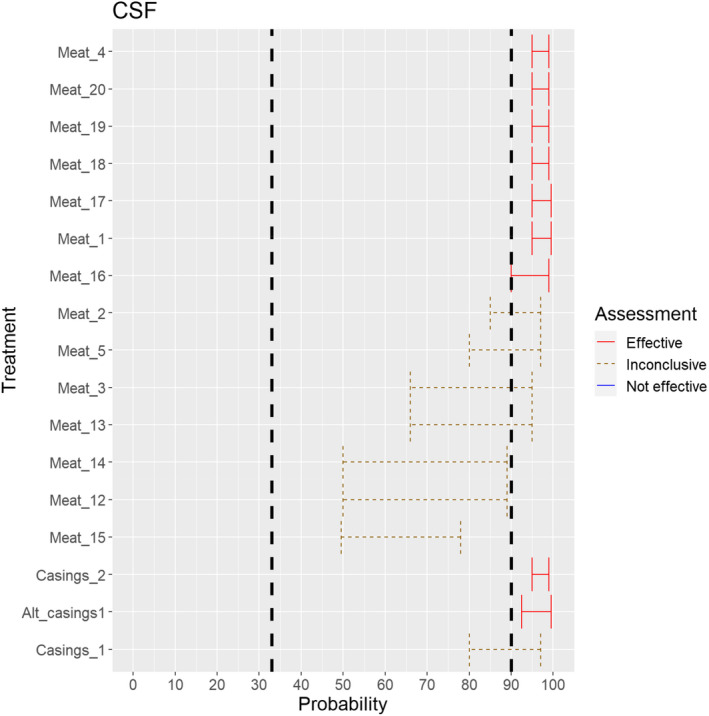
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the effectiveness of treatments for animal products to mitigate the risk for CSF virus (the vertical lines represent cut‐offs for categorisation of treatments as not effective (< 33%), inconclusive (33% to < 90% and medians spanning two or more areas) or effective (90–100%))
The ELS results were based on 10 references, which looked at experimental effects of the treatments on the CSFV in particular products. There were five publications relating to curing meat from infected animals and five related to inactivation of the virus in casings. Therefore, the evidence is incomplete and several of the assessments are based on the general properties of the virus and the WOAH Terrestrial animal health code (WOAH, 2021) or EU legislation.
The following 12 treatments from Annex VII or the ELS were considered effective:
-
•
Heat treatment in a hermetically sealed container, to achieve a minimum F0 value of 3 (Treatment Meat_1).
-
•
Heat treatment (to meat previously de‐boned and defatted) to achieve a core temperature of 70°C for a minimum of 30 min (Treatment Meat_4).
-
•
Drying after salting Italian style bone‐in hams: minimum 313 days (Treatment Meat_16).
-
•
Drying after salting Iberian hams: minimum 252 days (Treatment Meat_17).
-
•
Drying after salting Iberian shoulders: minimum 140 days (Treatment Meat_18).
-
•
Drying after salting Iberian loins: minimum 126 days (Treatment Meat_19).
-
•
Drying after salting Serrano hams: minimum 140 days (Treatment Meat_20).
-
•
Salting with phosphate supplemented salt 86.5% NaCl, 10.7% Na2HPO4 and 2.8% Na3PO4 either dry or as saturated brine (Aw < 0.80) for a continuous period of 30 days or longer at an ambient temperature of 20°C or above (Treatment casings_2).
-
•
Salting with citrate‐supplemented salt 89.2% NaCl, 8.9% trisodium citrate dihydrate and 1.9% citric acid monohydrate (wt/wt/wt), with pH 4.5 for a continuous period of 30 days or longer at an ambient temperature of 20°C or above (Treatment Alt_casings1).
The following treatments were assessed as inconclusive:
-
•
Meat heat treatment to achieve a core temperature of 80°C (Treatment Meat_2): This treatment is considered very likely to be effective given the high temperature, but uncertainty remains due to the absence of a prescribed duration of time.
-
•
Meat heat treatment to achieve a core temperature of 70°C (Treatment Meat_3): This treatment is considered likely to be effective given the high temperature, but uncertainty remains due to the absence of a prescribed duration of time.
-
•
Meat treatment in a hermetically sealed container, applying 60°C for a minimum of 4 h (Treatment Meat_5): The exposure to this temperature for this extended time is considered likely to be effective. However, a degree of uncertainty remains given the lack of scientific evidence for this specific temperature–time combination.
-
•
Natural fermentation and maturation for bone‐in meat: minimum 9 months, to achieve maximum values of Aw of 0.93 and pH of 6 (Treatment Meat_12): This treatment is considered likely to be effective given the long duration. A degree of uncertainty remains however linked to the absence of specific scientific evidence for this pH to be effective. Moreover, the presence of the bone, which might not ferment, adds to the uncertainty.
-
•
Natural fermentation and maturation for de‐boned meat: minimum 9 months, to achieve maximum values of Aw of 0.93 and pH of 6 (Treatment Meat_13): This treatment is considered likely to be effective given the long duration, and moreover as the bones have been removed. A degree of uncertainty remains however, linked to the absence of specific scientific evidence for this pH to be effective.
-
•
Natural fermentation for loins: minimum 140 days to achieve maximum values of Aw of 0.93 and pH of 6 (Treatment Meat_14): This treatment could potentially be effective, but uncertainty remains due to lack of specific scientific evidence for the levels of pH and of Aw to be effective.
-
•
Natural fermentation for hams: minimum 190 days to achieve maximum values of Aw of 0.93 and pH of 66 (Treatment Meat 15): This treatment could potentially be effective, but uncertainty remains due to lack of specific scientific evidence for the levels of pH and of Aw to be effective.
-
•
Salting with sodium chloride (NaCl) either dry or as saturated brine (Aw < 0.80), for a continuous period of 30 days or longer at an ambient temperature of 20°C or above (Treatment casings_1): This treatment is considered likely to be effective, but with a degree of uncertainty due to the absence of evidence for this specific treatment; the available evidence is based on quantitative models rather than from experimental studies (Wieringa‐Jelsma et al., 2011).
3.2.1.8. African swine fever virus
A total of 12 treatments listed for ASFV in Annex VII and three new suggested treatments were assessed. Twelve of these treatments concerned meat products and two concerned casings. Overall, 12 treatments were considered effective and for three treatments there was inconclusive evidence (Figure 35).
Figure 35.
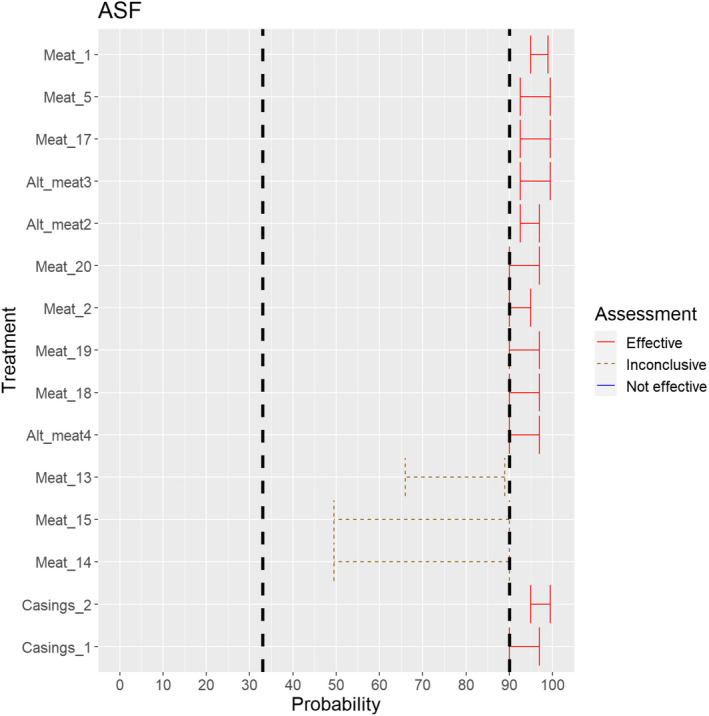
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the effectiveness of treatments for animal products to mitigate the risk for ASF virus (the vertical lines represent cut‐offs for categorisation of treatments as not effective (< 33%), inconclusive (33% to < 90% and medians spanning two or more areas) or effective (90–100%))
The ELS results were based on seven references which looked at experimental effects of the treatments on the ASFV in particular products. There were five publications relating to curing meat from infected animals and two related to inactivation of the virus in casings. Therefore, the evidence is incomplete and several of the assessments are based on the general properties of the virus and the WOAH Terrestrial animal health code (WOAH, 2021) or EU legislation.
The following 12 treatments were considered effective:
-
•
Meat heat treatment in a hermetically sealed container, to achieve a minimum F0 value of 3 (Treatment Meat_1).
-
•
Meat heat treatment to achieve a core temperature of 80°C (Treatment Meat_2).
-
•
Meat treatment in a hermetically sealed container, applying 60°C for a minimum of 4 h (Treatment Meat_5).
-
•
Drying after salting Iberian hams: minimum 252 days (Treatment Meat_17).
-
•
Drying after salting Iberian shoulders: minimum 140 days (Treatment Meat_18).
-
•
Drying after salting Iberian loins: minimum 126 days (Treatment Meat_19).
-
•
Drying after salting Serrano hams: minimum 140 days (Treatment Meat_20).
-
•
Meat heat treatment to achieve a core temperature of 70°C for at least 30 min (Treatment Alt_meat2).
-
•
Drying after salting Italian style bone‐in hams: minimum 400 days (Treatment Alt_meat3).
-
•
Drying after salting (Italian style) loins: minimum 137 days (Treatment Alt_meat4).
-
•
Casing salting with sodium chloride (NaCl) either dry or as saturated brine (Aw < 0.80), for a continuous period of 30 days or longer at an ambient temperature of 20°C or above (Treatment casings_1).
-
•
Casing salting with phosphate supplemented salt 86.5% NaCl, 10.7% Na2HPO4 and 2.8% Na3PO4 either dry or as saturated brine (Aw < 0.80) for a continuous period of 30 days or longer at an ambient temperature of 20°C or above (Treatment casings_2).
The following treatments were assessed as inconclusive:
-
•
Natural fermentation and maturation for de‐boned meat: minimum 9 months, to achieve maximum values of Aw of 0.93 and pH of 6 (Treatment Meat_13): This treatment is considered to probably be effective given the long duration and, moreover, as the bones have been removed. A degree of uncertainty remains however, linked to the absence of specific scientific evidence for this pH to be effective.
-
•
Natural fermentation for loins: minimum 140 days to achieve maximum values of Aw of 0.93 and pH of 6 (Treatment Meat_14): This treatment could potentially be effective, but uncertainty remains due to lack of specific scientific evidence for this pH and Aw to be effective.
-
•
Natural fermentation for loins: minimum 140 days to achieve maximum values of Aw of 0.93 and pH of 6 (Treatment Meat_15): This treatment could potentially be effective, but uncertainty remains due to lack of specific scientific evidence for this pH and Aw to be effective.
3.2.1.9. Highly pathogenic avian influenza virus
A total of 19 treatments listed for HPAIV in Annex VII and four new suggested treatments were assessed. Ten of these treatments concerned meat products and the remaining 13 concerned egg products. Overall, 21 treatments were considered effective, two treatments were considered ineffective and for one treatment there was inconclusive evidence (Figure 36).
Figure 36.
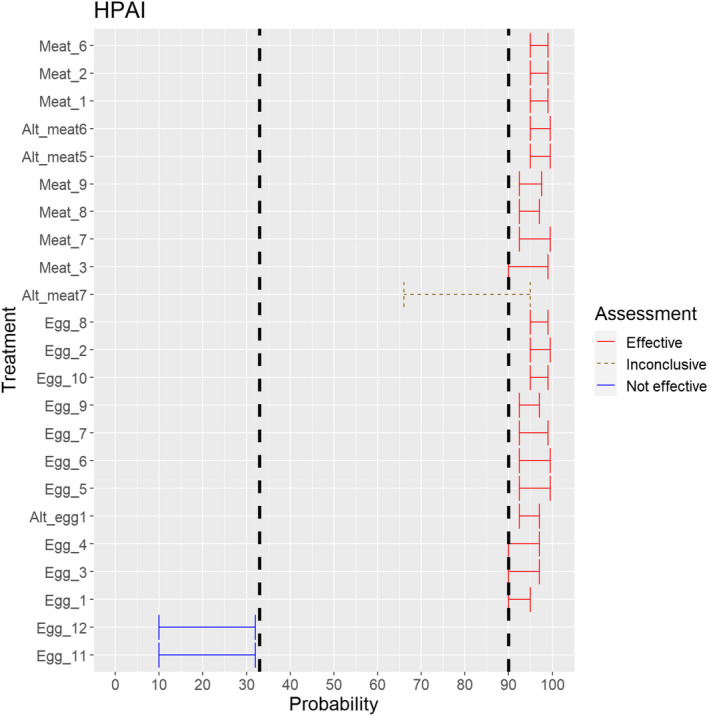
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the effectiveness of treatments for animal products to mitigate the risk for HPAI virus (the vertical lines represent cut‐offs for categorisation of treatments as not effective (< 33%), inconclusive (33% to < 90% and medians spanning two or more areas) or effective (90–100%))
The assessments were based on 16 references, from reports, reviews and original research assessing the effects of different treatments in meat and egg products on the inactivation of HPAIV.
The experts considered the following treatments as effective:
-
•
Meat heat treatment in a hermetically sealed container, to achieve a minimum F0 value of 3 (Meat_1).
-
•
Meat heat treatment to achieve a core temperature of 80°C (Treatment Meat_2).
-
•
Meat heat treatment to achieve a core temperature of 70°C (Treatment Meat_3).
-
•
Meat heat Core temperature of 73.9°C for a minimum of 0.51 s (Treatment Meat_6).
-
•
Core temperature of 70.0°C for a minimum of 3.5 s (Treatment Meat_7).
-
•
Core temperature of 65.0°C for a minimum of 42 s (Treatment Meat_8).
-
•
Core temperature of 60.0°C for a minimum of 507 s (Treatment Meat_9).
-
•
Core temperature of 70 °C for a minimum of 5 s (Treatment Alt_meat5).
-
•
Core temperature of 60 °C for a minimum of 60 min (Treatment Alt_meat6).
-
•
Whole egg: 60°C ‐ 188 s (Treatment Egg_1).
-
•
Whole egg: completely cooked (Treatment Egg_2).
-
•
Whole egg blends: 60°C ‐ 188 s (Treatment Egg_3).
-
•
Whole egg blends: 61.1°C ‐ 94 s (Treatment Egg_4).
-
•
Whole egg blends: completely cooked (Treatment Egg_5).
-
•
Liquid egg white: 55.6°C ‐ 870 s (Treatment Egg_6).
-
•
Liquid egg white: 56.7°C ‐ 232 s (Treatment Egg_7).
-
•
Plain or pure egg yolk: 60°C ‐ 288 s (Treatment Egg_8).
-
•
10% salted yolk: 62.2°C ‐ 138 s (Treatment Egg_9).
-
•
Dried egg white: 67°C ‐ 20 h (Treatment Egg_10).
The following treatments were considered not effective:
-
•
Dried egg white: 54.4°C ‐ 50.4 h (Treatment Egg_11): This treatment was considered not likely to be effective, based mostly on experimental data for temperatures 55, 57, 59, 61 and 63 C, resulting in D‐values of 2.2, 1.4, 1.3, 1.0 and 0.2 days, respectively reported by Swayne and Beck (2004). Moreover, it was shown that 21.38 days were needed to reach a 102 reduction at 54.4 C in dried egg white.
-
•
Dried egg white: 51.7°C ‐ 73.2 h (Treatment Egg_12): Similar to treatment Egg_11, this treatment was considered ineffective based on Swayne and Beck (2004).
The following treatment was assessed as inconclusive
-
•
Incubation at 500 MPa at 15 °C for a minimum of 15 s (Treatment Alt_meat7): This treatment could potentially be effective. However, the identified scientific evidence concerned the assessment of efficacy on meat suspension rather than meat and included also a pre‐treatment process (Isbarn et al., 2007), which led to a degree of uncertainty regarding this treatment.
3.2.1.10. Newcastle disease virus
A total of 16 treatments listed for NDV in Annex VII and 13 additional treatments identified by the ELS were assessed. Of these treatments, 13 concerned meat products and the remaining 16 concerned egg products. Overall, 21 treatments were considered by the experts as effective, three treatments were considered ineffective and for five treatments there were inconclusive evidence (Figure 37). The assessments were based on 10 references, from reports, reviews and original research assessing the effects of different treatments in meat and egg products on the inactivation of NDV.
Figure 37.
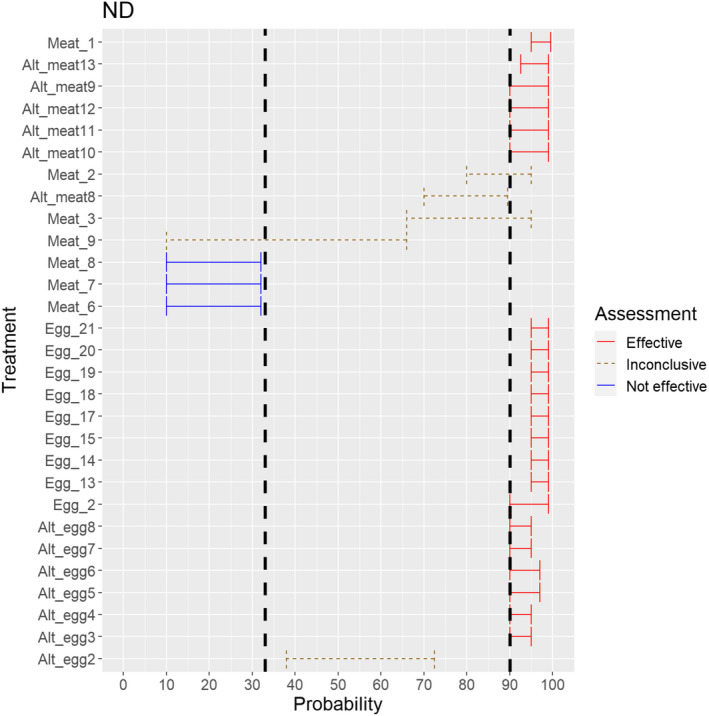
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the effectiveness of treatments for animal products to mitigate the risk for ND virus (the vertical lines represent cut‐offs for categorisation of treatments as not effective (< 33%), inconclusive (33% to < 90% and medians spanning two or more areas) or effective (90–100%))
Based on the ELS, the experts considered the following treatments to be effective:
-
•
Meat heat treatment in a hermetically sealed container, to achieve a minimum F0 value of 3 (Treatment Meat_1).
-
•
Meat core temperature of 70.0°C for a minimum of 82 s (Treatment Alt_meat9).
-
•
Meat core temperature of 74.0°C for a minimum of 40 s (Treatment Alt_meat10).
-
•
Meat core temperature of 80.0°C for a minimum of 29 s (Treatment Alt_meat11).
-
•
Meat core temperature above 70°C for a minimum of 82 s (Treatment Alt_meat12).
-
•
Meat core temperature of 57.8°C for a minimum of 63.3 min (Treatment Alt_meat13).
-
•
Whole egg: completely cooked (Treatment Egg_2).
-
•
Whole egg: 55°C ‐ 2521 s (Treatment Egg_13).
-
•
Whole egg: 57°C ‐ 1596 s (Treatment Egg_14).
-
•
Whole egg: 59°C ‐ 674 s (Treatment Egg_15).
-
•
Liquid egg white: 55°C ‐ 2278 s (Treatment Egg_17).
-
•
Liquid egg white: 57°C ‐ 986 s (Treatment Egg_18).
-
•
Liquid egg white: 59°C ‐ 301 s (Treatment Egg_19).
-
•
10% salted egg yolk: 55°C ‐ 176 s (Treatment Egg_20).
-
•
Dried egg white: 57°C ‐ 54.0 h (Treatment Egg_21).
-
•
Fortified egg: 61.1°C ‐ 6.2 min (Treatment Alt_egg3).
-
•
Fortified egg: 62.2°C ‐ 3.5 min (Treatment Alt_egg4).
-
•
Sugared/salted egg: 62.2°C ‐ 6.2 min (Treatment Alt_egg5).
-
•
Sugared/salted egg: 63.3°C ‐ 3.5 min (Treatment Alt_egg6).
-
•
Plain yolk: 60°C ‐ 6.2 min (Treatment Alt_egg7).
-
•
Plain yolk: 61.1°C ‐ 3.5 min (Treatment Alt_egg8).
The following treatments were considered not to be effective:
-
•
Meat core temperature of 73.9°C for a minimum of 0.51 s (Treatment Meat_6): Based on Alexander and Manvell (2004) it was considered that the duration of the temperature treatment was too short, with uncertainty on this treatment arising from the absence of specific scientific evidence.
-
•
Meat core temperature of 70.0°C for a minimum of 3.5 s (Treatment Meat_7): Based on the scientific evidence provided by the ELS (Alexander and Manvell, 2004), the duration of the temperature treatment is considered too short for the treatment to be effective.
-
•
Meat core temperature of 65.0°C for a minimum of 42 s (Treatment Meat_8): Based on the scientific evidence provided by the ELS (Alexander and Manvell, 2004), the duration of the temperature treatment is considered too short for the treatment to be effective.
The following treatments were assessed as inconclusive:
-
•
Meat heat treatment to achieve a core temperature of 80°C (Treatment Meat_2): This treatment could be potentially effective. However, there is no information of the required duration of the treatment which made assessment of this treatment inconclusive.
-
•
Meat heat treatment to achieve a core temperature of 70°C (Treatment Meat_3): The assessment of this treatment followed the same arguments as those provided for treatment Meat_2.
-
•
Meat core temperature of 60.0°C for a minimum of 507 s (Treatment Meat_9): The relationship between temperature duration of treatment and inactivation of the virus is not linear and with lower temperatures, it would be expected that longer time than the one prescribed would be needed. There was a large degree of uncertainty about this treatment due to absence of specific scientific evidence.
-
•
Meat core temperature of 65.0°C for a minimum of 120 s (Treatment Alt_meat8): There was a large degree of uncertainty about the effectiveness of this temperature–time combination due to absence of specific scientific evidence.
3.2.2. Assessment of the effectiveness of risk‐mitigating treatments for products not of animal origin from the protection zone (listed in Annex VIII of the DR)
3.2.2.1. Foot and mouth disease virus
No data were available for the treatment of feed materials of plant origin and straw for FMDV. Therefore, the evidence is incomplete, and the assessment was based on the general properties of the virus in culture, the environment, and the WOAH Terrestrial animal health code (WOAH, 2021).
Heat treatment, minimum temperature of 80°C and for a minimum of 10 min, steam in a closed chamber (Treatment Non_anim_prod1) was considered inconclusive (Figure 38). While core temperatures of 80°C are considered to be probably sufficient to inactivate the virus, no specific evidence referring to these products has been identified.
Figure 38.
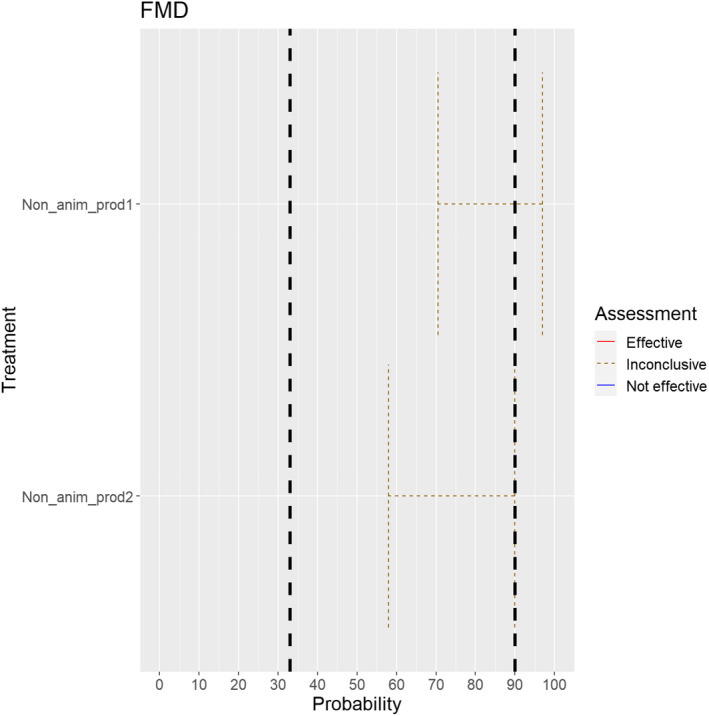
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the effectiveness of treatments for non‐animal products to mitigate the risk for FMD virus (the vertical lines represent cut‐offs for categorisation of treatments as not effective (< 33%), inconclusive (33% to < 90% and medians spanning two or more areas) or effective (90–100%))
Storage in package or bales under shelter at premises situated not closer than 2 km to the nearest outbreak and releasing from the premises do not take place before at least 3 months have elapsed following the completion of cleaning and disinfection according to Article 15 (Treatment Non_anim_prod2) was considered inconclusive as no specific evidence referring to these products has been identified (Figure 38). WOAH recommends a storage period of at least 4 months (WOAH, 2021).
3.2.2.2. Rinderpest virus
Two treatments listed for RPV in Annex VIII were assessed. Both treatments were considered effective (Figure 39).
Figure 39.
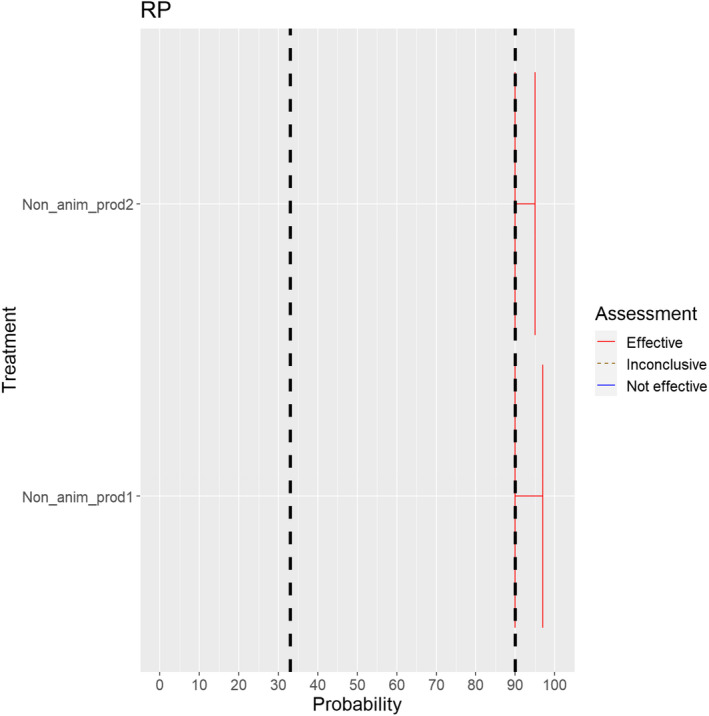
Median of the lower and upper bounds of the subjective probability ranges (in %) expressed by individual experts in the group discussion regarding the effectiveness of treatments for non‐animal products to mitigate the risk for RP virus (the vertical lines represent cut‐offs for categorisation of treatments as not effective (< 33%), inconclusive (33% to < 90% and medians spanning two or more areas) or effective (90–100%))
The ELS results included no data for the treatment of feed materials of plant origin and straw. Therefore, the evidence is incomplete, and the assessment was based on the general properties of the virus in culture, the environment and the WOAH Terrestrial animal health code (WOAH, 2021) or EU legislation.
For Heat treatment, minimum temperature of 80°C and for a minimum of 10 min, steam in a closed chamber (Treatment Non_anim_prod1) and Storage in package or bales under shelter at premises situated not closer than 2 km to the nearest outbreak and releasing from the premises do not take place before at least 3 months have elapsed following the completion of cleaning and disinfection according to Article 15 (Treatment Non_anim_prod2), the experts considered that these are effective treatments, because RPV is not very stable in the environment; still, some uncertainty exists due to lack of specific scientific evidence and the large range of materials included in this product category.
For RPV, there were no additional products for which the experts considered that treatment processes would be required to ensure safe trade.
4. Conclusions
The conclusions regarding the prohibitions of activities concerning animals and products related to Category A diseases in Annex VI of the DR and the effectiveness of risk mitigation treatments to control the presence of Category A disease agents in products of animal origin and other relevant materials listed in Annexes VII and VIII of the DR and additional risk‐mitigating treatments identified through the ELS are shown in a tabular format. The tables display the group ranges reflecting the individual judgements provided by the experts expressed by the experts. Assessments that concur with the current prohibitions or treatments prescribed in the Annexes of the DR are shown in green, assessments that diverge from the current Annexes are shown in yellow. Assessments that were inconclusive are displayed in blue. The reasons for divergence are provided in Sections 3.1 and 3.2 of this opinion, and recommendations regarding prohibitions and treatments are provided in the Recommendations section.
4.1. Assessment of prohibitions of activities concerning movements of animals related to Category A diseases in Annex VI of the DR (ToR 4.1)
The experts assessed the possibility of spread of the causative agents of the Category A diseases as a result of the movements of animals listed in Annex VI listed in Annex VI of the DR as shown in Table 7.
Table 7.
Conclusions regarding the possibility of spread of the causative agents of the Category A diseases as a result of the movements of animals listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (median of the lower and upper bounds of the subjective probability ranges (in %); green = confirmatory, blue = inconclusive, yellow = negative, grey = not applicable)
| FMD | RP | RVF* | LSD* | CBPP | SPGP | PPR | CCPP | CSF | ASF | AHS* | HPAI | ND | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Movements of kept animals from establishments in the restricted zone | 99–100 | 99–100 | 95–100 | 95–100 | 99–100 | 99–100 | 99–100 | 99–100 | 99–100 | 99–100 | 95–100 | 99–100 | 99–100 |
| Movements of kept animals to establishments in the restricted zone | 99–100 | 99–100 | 95–100 | 95–100 | 99–100 | 99–100 | 99–100 | 99–100 | 99–100 | 99–100 | 95–100 | 99–100 | 99–100 |
| Restocking of game animals | 99–100 | 99–100 | 95–100 | 95–100 | 99–100 | 99–100 | 99–100 | 99–100 | 99–100 | 99–100 | 95–100 | 99–100 | 99–100 |
| Fairs, markets, shows and other gatherings of kept animals including collection and dispersion of those species | 99–100 | 99–100 | 95–100 | 95–100 | 99–100 | 99–100 | 99–100 | 99–100 | 99–100 | 99–100 | 95–100 | 99–100 | 99–100 |
| Itinerant natural service of kept animals | 99–100 | 99–100 | 95–100 | 95–100 | 99–100 | 99–100 | 99–100 | 99–100 | 99–100 | 99–100 | 95–100 |
In the case of vector‐borne diseases, transmission is intended to include vector‐borne transmission of the disease agent.
4.2. Assessment of prohibitions of activities concerning movements of germinal products related to Category A diseases in Annex VI of the DR (ToR 4.1)
The experts assessed the possibility that the germinal products listed in Annex VI of the DR collected or derived from infected animals of listed species can contain the infectious disease agent as shown in Table 8.
Table 8.
Conclusions regarding the possibility of spread of the causative agents of the Category A diseases as a result of the activities regarding germinal products listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (median of the lower and upper bounds of the subjective probability ranges (in %); green = confirmatory, blue = inconclusive, yellow = negative, grey = not applicable)
| Can the germinal product collected or derived from infected animals of listed species contain the infectious disease agent? | FMD | RP | RVF | LSD | CBPP | SPGP | PPR | CCPP | CSF | ASF | AHS | HPAI | ND |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Semen | 90–100 | 85–100 | 90–100 | 95–100 | 70–100 | 10–99 | 33–100 | 33–95 | 95–100 | 80–100 | 66–100 | 90–100 | 90–100 |
| Oocytes | 0–66 | 1–66 | 10–90 | 33–100 | 50–100 | 5–99 | 10–95 | 33–99 | 33–99 | 10–95 | 10–90 | ||
| In vivo derived embryos | 1–50 | 0–50 | 10–90 | 33–100 | 66–100 | 5–99 | 10–95 | 33–95 | 10–75 | 66–100 | 0–90 | ||
| In vitro produced embryos | 33–95 | 1–50 | 10–89.9 | 33–100 | 66–100 | 5–99 | 10–95 | 50–95 | 10–95 | 66–100 | 0–99 | ||
| Hatching eggs | 70–100 | 70–100 |
There is a lack of evidence to allow making conclusions with high degree of certainty on the possibility that germinal products collected or derived from an infected animal of the listed species (i.e. kept animals, and, where relevant, game animals) in the restricted zone can contain the disease agent, particularly regarding oocytes, in vivo derived and in vitro produced embryos.
At present, the collection of semen from animals in AHS restricted areas is not prohibited in Annex VI. WOAH states that the virus can be present in semen of viraemic animals (WOAH, 2021). However, even though the disease is vector‐borne, evidence that there is no potential to transmit the disease agent through contaminated semen is needed.
New scientific evidence on the presence of infectious ASF virus in semen of infected boars substantiates the current prohibitions regarding the movement of semen.
At present, the movement of semen from animals in LSD restricted areas is not prohibited in Annex VI. Scientific evidence shows that the virus is present in semen of infected cattle. It has been shown that cows inseminated with LSDV‐spiked semen can become infected and the embryos harvested from these cows can become externally contaminated. To what extent this can be extrapolated to naturally contaminated semen remains uncertain.
At present, the collection and movement of semen from animals in HPAI and ND restricted areas is not prohibited in Annex VI. Scientific evidence shows that these viruses can be present in semen of infected poultry (Pantin‐Jackwood and Swayne, 2009; Dhama et al., 2014; Cardona et al., 2021).
Although internationally accepted guidelines exist (e.g. IETS), no standardised rules for the safe collection of germinal products for domestic use exist. Their adoption also for domestic use could potentially reduce the possibility that disease agent is present in the germinal products after their collection.
4.3. Assessment of prohibitions of activities concerning movements of animal products, animal by‐products and movements of feed of plant origin and straw related to Category A diseases in Annex VI of the DR (ToR 4.1)
The experts assessed the possibility of spread of the causative agents of the Category A diseases as a result of the movements of animal products, animal by‐products and movements of feed of plant origin and straw listed in Annex VI as shown in Table 9.
Table 9.
Conclusions regarding the possibility of spread of the causative agents of the Category A diseases as a result of the movements of animal products, animal by‐products and movements of feed of plant origin and straw listed in Annex VI, Commission Delegated Regulation (EU) 2020/687 (median of the lower and upper bounds of the subjective probability ranges (in %); green = confirmatory, blue = inconclusive, yellow = negative, grey = not applicable)
| Prohibitions of activities concerning movements of animal products, animal by‐products and feed of plant origin and straw related to Category A diseases | FMD | RP | RVF* | LSD* | CBPP | SPGP | PPR | CCPP | CSF | ASF | AHS* | HPAI | ND |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Movements of fresh meat excluding offal from kept and wild animals of listed species from slaughterhouses or game handling establishments in the restricted zone | 93–100 | 66–95 | 70–95 | 22–50 | 0–10 | 1–33 | 66–95 | 3–15 | 97–100 | 97–100 | 0–5 | 95–99 | 97–100 |
| Movements of offal from kept and wild animals of listed species from slaughterhouses or game handling establishments in the restricted zone | 95–100 | 78–99 | 70–95 | 33–66 | 38–90 | 17–50 | 66–95 | 50–90 | 97–100 | 97–100 | 0–5 | 95–99 | 97–100 |
| Movements of meat products obtained from fresh meat of listed species from establishments in the restricted zone | 85–100 | 33–90 | 1–33 | 1–23 | 0–10 | 1–15 | 50–95 | 0–10 | 95–100 | 97–100 | 0–5 | 95–100 | 97–100 |
| Movements of raw milk and colostrum obtained from kept animals of listed species from establishments in the restricted zone | 95–100 | 66–92 | 66–99 | 70–88 | 0–10 | 42–73 | 66–99 | 0–10 | 0–5 | ||||
| Movements of dairy products and colostrum‐based products from establishments in the restricted zone | 85–100 | 66–90 | 66–90 | 58–85 | 0–8 | 42–73 | 58–90 | 0–5 | 0–5 | ||||
| Movements of eggs for human consumption from establishments in the restricted zone | 81–95 | 85–98 | |||||||||||
| Movements of animal by‐products from kept animals of listed species from establishments in the restricted zone, except entire bodies or parts of dead animals: manure, including litter and used bedding | 93–100 | 58–95 | 17–51 | 22–54 | 3–10 | 50–83 | 55–90 | 0–8 | 90–100 | 90–100 | 0–1 | 93–100 | 93–99 |
| Movements of animal by‐products from kept animals of listed species from establishments in the restricted zone, except entire bodies or parts of dead animals: hides, skins, wool, bristles and feathers | 93–100 | 33–66 | 8–43 | 80–95 | 3–10 | 80–95 | 33–90 | 0–10 | 78–99 | 88–95 | 0–1 | 95–100 | 90–100 |
| Movements of animal by‐products from kept animals of listed species from establishments in the restricted zone, except entire bodies or parts of dead animals: animal by‐products other than manure, including litter and used bedding, and other than hides, skins, wool, bristles and feathers | 78–100 | 58–93 | 50–78 | 22–58 | 5–33 | 22–58 | 66–95 | 3–33 | 95–100 | 97–100 | 0–5 | 95–100 | 95–100 |
| Movements of feed material of plant origin and straw obtained in the protection zone | 75–97 | 45–73 | 0–20 | 3–27 | 0–10 | 66–90 | 29–66 | 0–10 | 50–90 | 61–90 | 0–1 | 90–100 | 90–99 |
There is a lack of evidence from observational studies in endemic areas to allow making conclusions with high degree of certainty on the role of animal products, animal by‐products and feed of plant origin and straw in epidemics.
The assessment of the risk associated with movements of meat products has a large degree of uncertainty due to the large variation of production processes and the likelihood of exposure to susceptible animals.
In general, the observed uncertainty related to the assessments of the possibility that the disease agents can be spread via the movement of feed is due to the wide range of different feed materials and the lack of scientific evidence on survival of the disease agents in these. It was concluded that a particular risk is associated with local movements of feed material of plant origin and straw obtained in the protection zone that has been contaminated by infected livestock, and, where relevant, by infected wildlife, during production or storage.
4.4. Assessment of the effectiveness of risk mitigation treatments to control the presence of Category A disease agents in products of animal origin and other relevant materials listed in Annexes VII and VIII of the DR and additional risk‐mitigating treatments identified through the ELS (ToR 4.2)
The experts the effectiveness of risk mitigation treatments for the control of Category A diseases in products of animal origin and other relevant material listed in Annexes VII and VIII of the DR as shown in Tables 10 and 11. The assessment of additional risk mitigation treatments identified through the ELS are shown in Table 12.
Table 10.
Conclusions regarding the effectiveness of risk mitigation treatments for the control of Category A diseases in products of animal origin (median of the lower and upper bounds of the subjective probability ranges (in %); green = effective, blue = inconclusive, yellow = not effective, empty cells = no assessment requested)
| Product | Assessed risk‐mitigating treatments for products of animal origin listed in Annex VII (ToR 4.2a) | Short name | FMD | RP | RVF | LSD | CBPP | SPGP | PPR | CCPP | CSF | ASF | AHS | HPAI | ND |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meat | Heat treatment in a hermetically sealed container, to achieve a minimum F0 value of 3 | Meat_1 | 97–100 | 95–100 | 95–100 | 95–99 | 95–99 | 95–100 | |||||||
| Heat treatment to achieve a core temperature of 80°C | Meat_2 | 66–90 | 90–99 | 85–97 | 90–97 | 95–99 | 80–95 | ||||||||
| Heat treatment to achieve a core temperature of 70°C | Meat_3 | 50–90 | 70–99 | 90–99 | 66–95 | 90–99 | 66–95 | ||||||||
| Heat treatment (to meat previously de‐boned and defatted) to achieve a core temperature of 70°C for a minimum of 30 min | Meat_4 | 97–100 | 95–100 | 95–99 | |||||||||||
| In a hermetically sealed container, applying 60°C for a minimum of 4 h | Meat_5 | 33–78 | 93–100 | 80–97 | 93–100 | ||||||||||
| Core temperature of 73.9 °C for a minimum of 0.51 s | Meat_6 | 33–66 | 95–99 | 10–32 | |||||||||||
| Core temperature of 70.0°C for a minimum of 3.5 s | Meat_7 | 93–100 | 10–32 | ||||||||||||
| Core temperature of 65.0°C for a minimum of 42 s | Meat_8 | 93–97 | 10–32 | ||||||||||||
| Core temperature of 60.0°C for a minimum of 507 s | Meat_9 | 93–98 | 10–66 | ||||||||||||
| Heat treatment to achieve a core temperature of 65°C for a period of time to achieve a minimum pasteurisation value of 40 | Meat_11 | 95–99 | |||||||||||||
| Natural fermentation and maturation for bone‐in meat: minimum 9 months, to achieve maximum values of Aw of 0.93 and pH of 6 | Meat_12 | 71–95 | 50–89 | ||||||||||||
| Natural fermentation and maturation for de‐boned meat: minimum 9 months, to achieve maximum values of Aw of 0.93 and pH of 6 | Meat_13 | 93–98 | 66–95 | 66–89 | |||||||||||
| Natural fermentation for loins: minimum 140 days to achieve maximum values of Aw of 0.93 and pH of 6 | Meat_14 | 50–89 | 50–90 | ||||||||||||
| Natural fermentation for hams: minimum 190 days to achieve maximum values of Aw of 0.93 and pH of 6 | Meat_15 | 50–78 | 50–90 | ||||||||||||
| Drying after salting Italian style bone‐in hams: minimum 313 days | Meat_16 | 90–99 | |||||||||||||
| Drying after salting Iberian hams: minimum 252 days | Meat_17 | 95–100 | 95–100 | 93–100 | |||||||||||
| Drying after salting Iberian shoulders: minimum 140 days | Meat_18 | 95–100 | 95–99 | 90–97 | |||||||||||
| Drying after salting Iberian loins: minimum 126 days | Meat_19 | 95–100 | 95–99 | 90–97 | |||||||||||
| Drying after salting Serrano hams: minimum 140 days | Meat_20 | 33–66 | 95–99 | 90–97 | |||||||||||
| Maturation of carcasses at a minimum temperature of 2°C for a minimum of 24 h following slaughter | Meat_21 | 50–88 | |||||||||||||
| Removal of offal | Meat_22 | 33–89 | 90–99 | ||||||||||||
| Casings | Salting with sodium chloride (NaCl) either dry or as saturated brine (Aw < 0.80), for a continuous period of 30 days or longer at an ambient temperature of 20°C or above | Casings_1 | 97–100 | 85–99 | 80–97 | 90–97 | |||||||||
| Salting with phosphate supplemented salt 86.5% NaCl, 10.7% Na2HPO4 and 2.8% Na3PO4 either dry or as saturated brine (Aw < 0 .80) for a continuous period of 30 days or longer at an ambient temperature of 20°C or above | Casings_2 | 97–100 | 85–99 | 95–99 | 95–100 | ||||||||||
| Milk | Heat treatment (sterilisation process) to achieve a minimum F0 value of 3 | Milk_1 | 95–99 | ||||||||||||
| Heat treatment UHT (Ultra‐high temperature): Minimum 132°C for a minimum of 1 s | Milk_2 | 42–90 | 66–95 | ||||||||||||
| Heat treatment UHT (Ultra‐high temperature): Minimum 135°C for a suitable holding time | Milk_3 | 42–85 | |||||||||||||
| Heat treatment HTST (High‐temperature short‐time) pasteurisation if milk pH is lower than 7, minimum 72°C for a minimum of 15 s | Milk_4 | 10–32 | 33–80 | ||||||||||||
| Heat treatment HTST (High‐temperature short‐time) pasteurisation if milk pH is 7 or higher, minimum 72°C for a minimum of 15 s, applied twice | Milk_5 | 42–78 | 90–99 | ||||||||||||
| Heat treatment HTST (High‐temperature short‐time) pasteurisation combined with a physical treatment to achieve pH value below 6 for a minimum of 1 h or Heat treatment HTST to achieve a minimum of 72°C, combined with desiccation | Milk_6 | 50–89 | |||||||||||||
| Pasteurisation consisting in a single heat treatment with an effect at least equivalent to that achieved by applying 72°C for 15 s | Milk_7 | 10–32 | 33–66 | 33–66 | |||||||||||
| Eggs | Whole egg: 60°C ‐ 188 s | Egg_1 | 90–95 | ||||||||||||
| Whole egg: completely cooked | Egg_2 | 95–100 | |||||||||||||
| Whole egg blends: 60°C ‐ 188 s | Egg_3 | 90–97 | |||||||||||||
| Whole egg blends: 61.1°C ‐ 94 s | Egg_4 | 90–97 | |||||||||||||
| Whole egg blends: completely cooked | Egg_5 | 93–100 | |||||||||||||
| Liquid egg white: 55.6°C ‐ 870 s | Egg_6 | 93–100 | |||||||||||||
| Liquid egg white: 56.7°C ‐ 232 s | Egg_7 | 93–99 | |||||||||||||
| Plain or pure egg yolk: 60°C ‐ 288 s | Egg_8 | 95–99 | |||||||||||||
| 10% salted yolk: 62.2°C ‐ 138 s | Egg_9 | 93–97 | |||||||||||||
| Dried egg white: 67°C ‐ 20 h | Egg_10 | 95–99 | |||||||||||||
| Dried egg white: 54.4°C ‐ 50.4 h | Egg_11 | 10–32 | |||||||||||||
| Dried egg white: 51.7°C ‐ 73.2 h | Egg_12 | 10–32 | |||||||||||||
| Whole egg: 55°C – 2,521 s | Egg_13 | 95–99 | |||||||||||||
| Whole egg: 57°C ‐ 1,596 s | Egg_14 | 95–99 | |||||||||||||
| Whole egg: 59°C ‐ 674 s | Egg_15 | 95–99 | |||||||||||||
| Whole egg: completely cooked | Egg_16 | 90–99 | |||||||||||||
| Liquid egg white: 55°C – 2,278 s | Egg_17 | 95–99 | |||||||||||||
| Liquid egg white: 57°C ‐ 986 s | Egg_18 | 95–99 | |||||||||||||
| Liquid egg white: 59°C – 301 s | Egg_19 | 95–99 | |||||||||||||
| 10% salted egg yolk: 55°C ‐ 176 s | Egg_20 | 95–99 | |||||||||||||
| Dried egg white: 57°C ‐ 54.0 h | Egg_21 | 95–99 |
Table 11.
Conclusions regarding the effectiveness of risk mitigation treatments for the control of Category A diseases in products of non‐animal origin (median of the lower and upper bounds of the subjective probability ranges (in %); green = effective, blue = inconclusive, yellow = not effective)
| Product | Assessed risk‐mitigating treatments for products of non‐animal origin listed in Annex VIII (ToR 4.2a) | Short name used in figures and text | FMD | RP |
|---|---|---|---|---|
| Feed materials of plant origin and straw | Heat treatment, minimum temperature of 80°C and for a minimum of 10 min, steam in a closed chamber | Non_anim_prod1 | 71–97 | 90–97 |
| Storage in package or bales under shelter at premises situated not closer than 2 km to the nearest outbreak and releasing from the premises do not take place before at least three months have elapsed following the completion of cleaning and disinfection according to Article 15 | Non_anim_prod2 | 58–90 | 90–95 |
Table 12.
Conclusions regarding the effectiveness of additional risk mitigation treatments for the control of Category A diseases in products of animal origin identified by the ELS (median of the lower and upper bounds of the subjective probability ranges (in %); green = effective, blue = inconclusive, yellow = not effective, empty cells = not applicable)
| Product | Assessed risk‐mitigating treatments for products of animal origin identified by the ELS (ToR 4.2b) | Short name used in figures and text | FMD | RP | RVF | LSD | CBPP | SPGP | PPR | CCPP | CSF | ASF | AHS | HPAI | ND |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Casings | Salting with citrate‐supplemented salt 89.2% NaCl, 8.9% trisodium citrate dehydrate and 1.9% citric acid monohydrate (wt/wt/wt), with pH 4.5 for a continuous period of 30 days or longer at an ambient temperature of 20°C or above | Alt_casings1 | 93–100 | ||||||||||||
| Eggs | Dried egg white: 54.4°C ‐ 21.38 days | Alt_egg1 | 93–97 | ||||||||||||
| Liquid whole egg: 64.4°C – 200 s | Alt_egg2 | 38–73 | |||||||||||||
| Fortified egg: 61.1°C – 6.2 min | Alt_egg3 | 90–95 | |||||||||||||
| Fortified egg: 62.2°C – 3.5 min | Alt_egg4 | 90–95 | |||||||||||||
| sugared/salted egg: 62.2°C – 6.2 min | Alt_egg5 | 90–97 | |||||||||||||
| sugared/salted egg: 63.3°C – 3.5 min | Alt_egg6 | 90–97 | |||||||||||||
| plain yolk: 60°C – 6.2 min | Alt_egg7 | 90–95 | |||||||||||||
| plain yolk: 61.1°C – 3.5 min | Alt_egg8 | 90–95 | |||||||||||||
| Meat | Drying after salting Serrano hams: minimum 182 day | Alt_meat1 | 97–100 | ||||||||||||
| Heat treatment to achieve a core temperature of 70°C for at least 30 min | Alt_meat2 | 93–97 | |||||||||||||
| Drying after salting Italian style bone‐in hams: minimum 400 days | Alt_meat3 | 93–100 | |||||||||||||
| Drying after salting (Italian style) loins: minimum 137 days | Alt_meat4 | 90–97 | |||||||||||||
| Core temperature of 70 °C for a minimum of 5 s | Alt_meat5 | 95–100 | |||||||||||||
| Core temperature of 60 °C for a minimum of 60 min | Alt_meat6 | 95–100 | |||||||||||||
| Incubation at 500 MPa at 15 °C for a minimum of 15 s | Alt_meat7 | 66–95 | |||||||||||||
| Core temperature of 65.0°C for a minimum of 120 s | Alt_meat8 | 70–90 | |||||||||||||
| Core temperature of 70.0°C for a minimum of 82 s | Alt_meat9 | 90–99 | |||||||||||||
| Core temperature of 74.0°C for a minimum of 40 s | Alt_meat10 | 90–99 | |||||||||||||
| Core temperature of 80.0°C for a minimum of 29 s | Alt_meat11 | 90–99 | |||||||||||||
| Core temperature above 70°C for a minimum of 82 s | Alt_meat12 | 90–99 | |||||||||||||
| Core temperature of 57.8°C for a minimum of 63.3 min | Alt_meat13 | 93–99 | |||||||||||||
| Milk | Pasteurisation consisting in a single heat treatment with an effect at least equivalent to that achieved by applying 72°C for 15 s with additional acidification | Alt_milk1 | 66–95 | 80–99 | |||||||||||
| Heat treatment HTST (High‐temperature short‐time) pasteurisation combined with a physical treatment to achieve pH value below 6 for a minimum of 1 h or High temperature short time pasteurisation to achieve a minimum of 72°C, combined with desiccation | Alt_milk2 | 93–99 | |||||||||||||
| Heat treatment UHT (Ultra‐high temperature): Minimum 132°C for a minimum of 1 s, combined with another physical treatment | Alt_milk3 | 93–99 |
5. Recommendations
5.1. Prohibitions of activities concerning movements of animals
Regarding prohibitions of activities concerning animals related to Category A diseases listed in Annex VI of the DR (ToR 4.1), the following recommendations are made:
-
•
Irrespective of the purpose or nature of the activity, it is recommended to not move animals from restricted zones without appropriate mitigation measures. Appropriate mitigation measures have been assessed in previous EFSA opinions (EFSA AHAW Panel, 2021a, 2021b, 2021c, 2021d, 2021e, 2021f, 2021g, 2021h, 2022a, 2022b, 2022c, 2022d, 2022e, 2022f).
5.2. Prohibitions of activities concerning movements of germinal products
Based on the scientific evidence identified/available, it was not possible to conclude that the movement of germinal products in Table 8 would be safe with a ≥ 99% level of certainty. Therefore, to reduce the uncertainty, scientific evidence from well‐designed studies should be generated.
Until evidence is generated that there is no potential to transmit AHSV through contaminated semen, the prohibition of the collection of semen from animals in AHS restricted zones should also be considered.
Until evidence is generated that there is no risk to transmit LSDV through contaminated semen collected from an infected animal as opposed to spiked semen, the prohibition of the movement of semen from animals in LSD restricted zones should also be considered.
Until evidence is generated that there is no potential to transmit NDV through contaminated semen, the prohibition of the movement of semen from poultry in ND restricted zones should be considered.
Regarding FMD, prohibitions regarding movements of in vivo derived embryos of cattle could be reconsidered because for cattle scientific evidence indicates that the risk might be low.
As there is scientific evidence showing that there is a risk to transmit HPAIV through contaminated semen, the prohibition of the movement of semen from poultry in HPAI restricted zones should be considered.
5.3. Prohibitions of activities concerning movements of animal products, animal by‐products and movements of feed of plant origin and straw
With the exception of AHS, based on the scientific evidence identified, it was not possible to conclude that the movement of the animal products, animal by‐products and movements of feed of plant origin and straw in Table 9 would be safe with a ≥ 99% level of certainty. Therefore, to reduce the uncertainty, scientific evidence would be needed.
Table 13 Prohibitions of movements assessed as inconclusive regarding the risk of spreading the Category A diseases due to insufficient/inconclusive scientific evidence
| Prohibitions of activities concerning movements of animal products, animal by‐products and feed of plant origin and straw related to Category A diseases | Disease |
|---|---|
| Movements of fresh meat excluding offal from kept and wild animals of listed species from slaughterhouses or game handling establishments in the restricted zone | SPGP, AHS |
| Movements of offal from kept and wild animals of listed species from slaughterhouses or game handling establishments in the restricted zone | AHS |
| Movements of meat products obtained from fresh meat of listed species from establishments in the restricted zone | RVF, LSD, CBPP, SPGP, CCPP, AHS |
| Movements of raw milk and colostrum obtained from kept animals of listed species from establishments in the restricted zone | CBPP, CCPP, AHS |
| Movements of dairy products and colostrum‐based products from establishments in the restricted zone | CBPP, CCPP, AHS |
| Movements of animal by‐products from kept animals of listed species from establishments in the restricted zone, except entire bodies or parts of dead animals: manure, including litter and used bedding | CCPP |
| Movements of animal by‐products from kept animals of listed species from establishments in the restricted zone, except entire bodies or parts of dead animals: hides, skins, wool, bristles and feathers | CCPP |
| Movements of animal by‐products from kept animals of listed species from establishments in the restricted zone, except entire bodies or parts of dead animals: animal by‐products other than manure, including litter and used bedding, and other than hides, skins, wool, bristles and feathers | AHS |
| Movements of feed material of plant origin and straw obtained in the protection zone | RVF, CBPP, CCPP |
Regarding African Horse Sickness, the experts assessed the possibility that movements of the products (raw milk, dairy products, fresh meat, meat products, offal and by‐products) leads to spread of the disease as almost impossible to extremely unlikely, but due to the scarcity of scientific evidence there is uncertainty. To reduce the uncertainty, scientific evidence would be needed.
Evidence from well‐designed observational studies in endemic areas and systematic and thorough outbreak investigations should be generated to reduce the level of uncertainty regarding the role of products in the spread of the disease agent.
Regarding zoonotic diseases, e.g. RVF, it is recommended to consider the public health risk associated with movements of products, in addition to the animal health risk.
Currently movements of feed material of plant origin and straw obtained in the protection zone are not prohibited except for RP and FMD. Especially for those diseases for which the possibility was assessed as being much larger than 1%, it is recommended that the risk manager considers requiring licensing of movements of feed material of plant origin and straw out of the restriction zone.
5.4. Risk mitigation treatments for products of animal origin and other relevant materials
Regarding risk mitigation treatments to control the presence of Category A disease agents in products of animal origin and other relevant materials listed in Annexes VII and VIII of the DR (ToR 4.2 a and b), the following recommendations are made:
5.4.1. Complete the treatment description
For heat treatments, the temperature that must be reached in the core of the treated product and the duration for which that temperature needs to be maintained to be effective should be provided in the treatment description. In analogy, for treatments involving a change of pH, the pH that must be reached throughout the treated product and the duration for which that pH needs to be maintained to be effective should be provided. Treatments for which experts recommend that the description of the treatment be completed are listed in Appendix B.1. Should no scientific evidence exist for the missing information, it is recommended to carry out experimental studies to fill the knowledge gaps.
5.4.2. Need for additional scientific evidence
For risk mitigation treatments for which the identified scientific evidence has been assessed as inconclusive, additional scientific evidence should be generated, possibly through new experimental studies (Appendix B.2).
5.4.3. Treatments not recommended
For risk mitigation treatments that have been assessed as not effective based on the identified scientific evidence, the Panel recommends not to use them (Appendix B.3). Where available, alternative treatments identified through the ELS that have been assessed as effective for the disease agent should be applied (e.g. alternative meat treatments Alt_meat 9, 10, 11, 12, 13 for NDV).
5.4.4. Recommendation for treatments of feed materials of plant origin and straw
For the mitigation of the FMDV spread risk associated with feed materials of plant origin and straw, it is recommended to monitor that the temperature of 80°C is reached for 10 min throughout the material when applying Heat treatment, minimum temperature of 80°C and for a minimum of 10 min, steam in a closed chamber (Treatment Non_anim_prod1 4 ).
Further, it is recommended to apply the storage period of four months stipulated by WOAH for storage of feed materials of plant origin and straw in package or bales (Treatment Non_anim_prod2 5 ).
For those additional Category A diseases for which feed materials of plant origin and straw pose a risk of spread (e.g. ASF, see EFSA, 2020), including through vectors, it is recommended to assess the effectiveness of risk mitigation treatments.
Appendix A – Final individual assessment judgements
A.1. Final individual judgements of the assessment of prohibitions of movements of animals
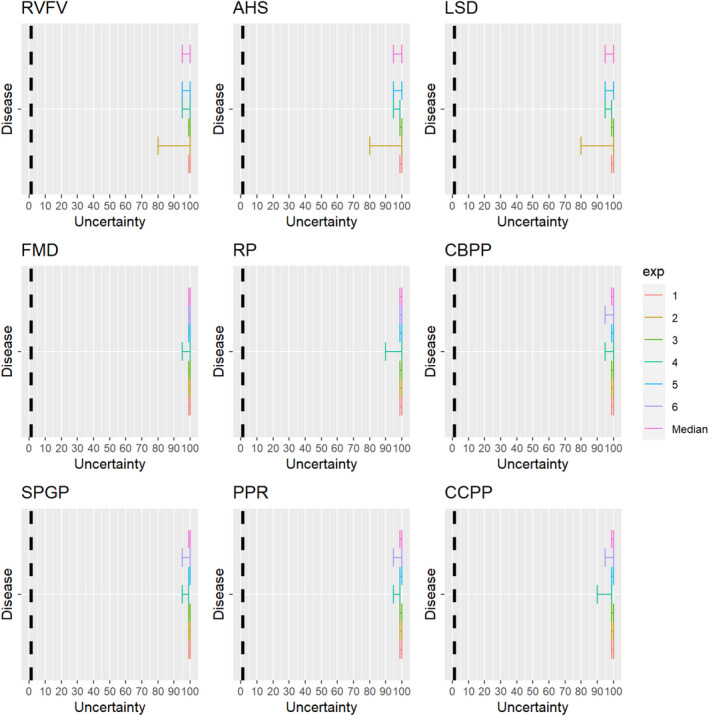
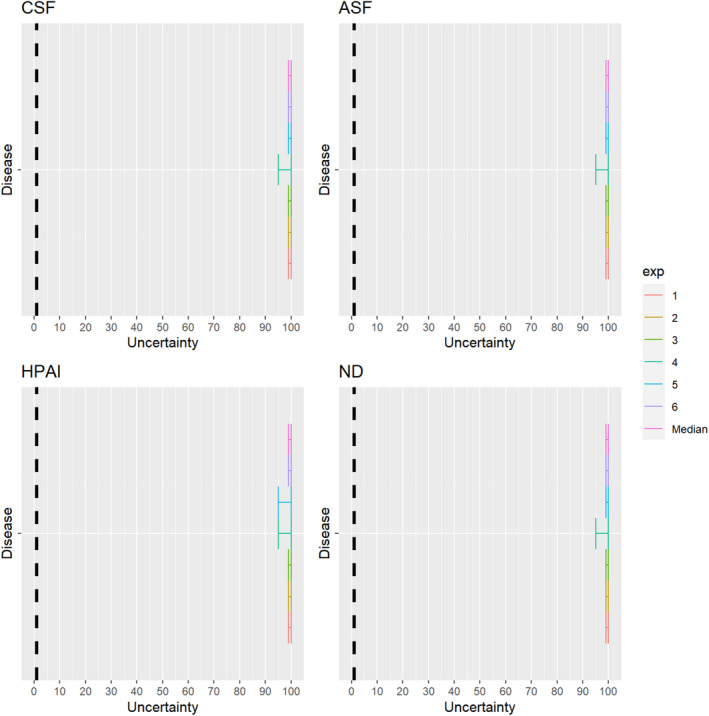
A.2. Final individual judgements of the assessment of prohibitions of movements of germinal products
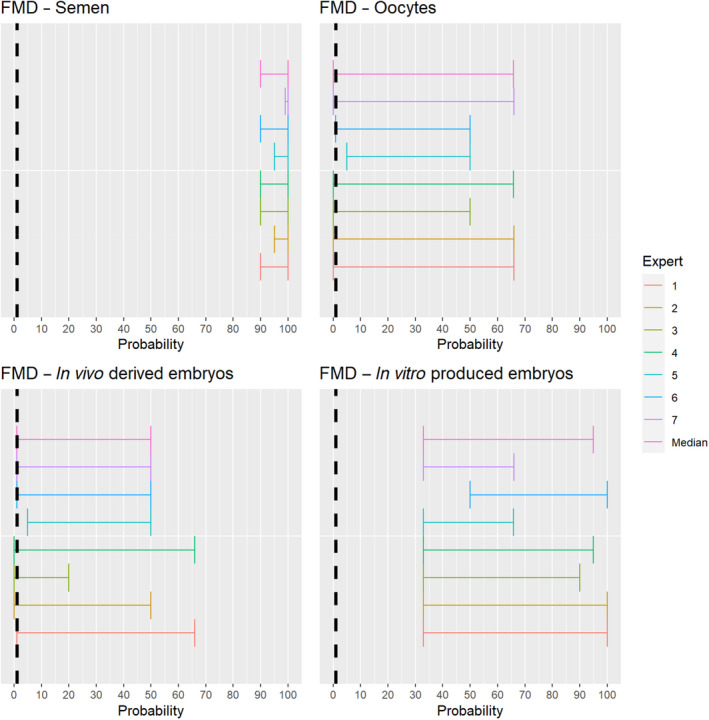
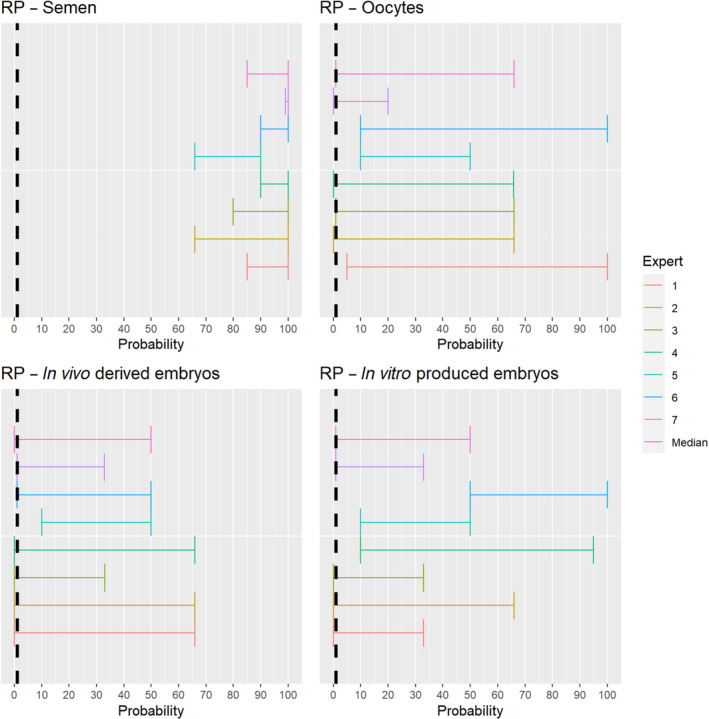
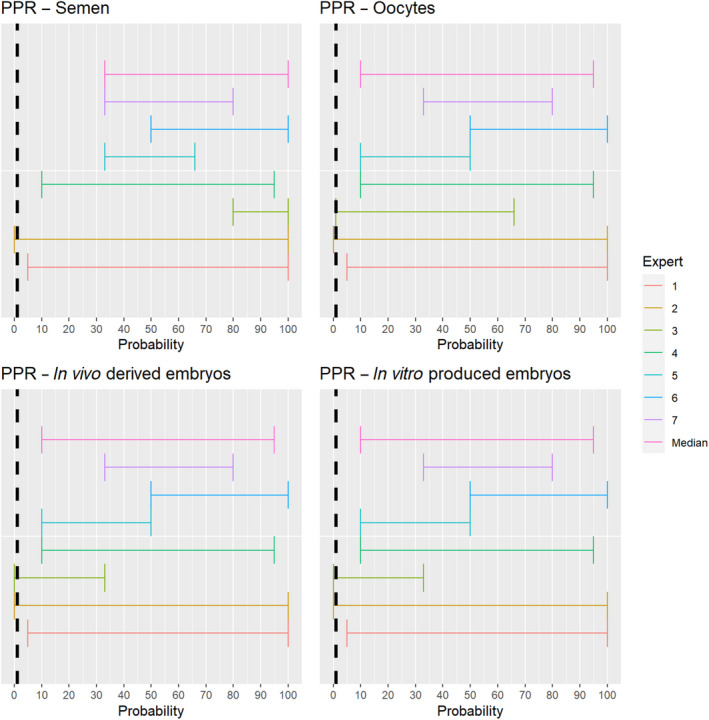
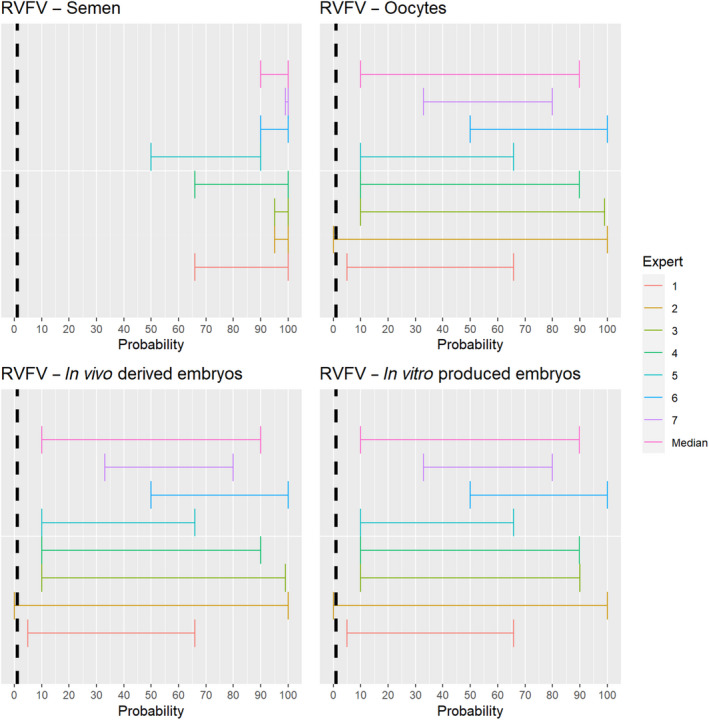
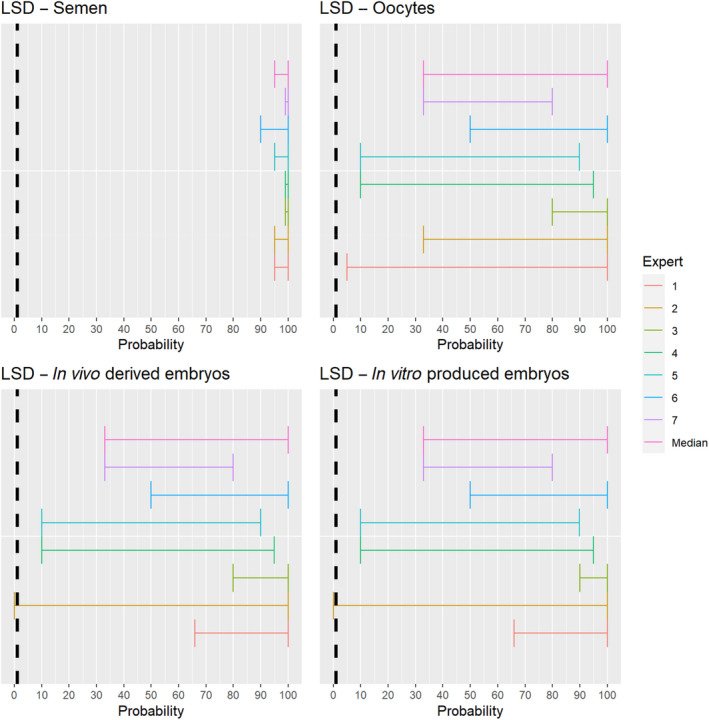
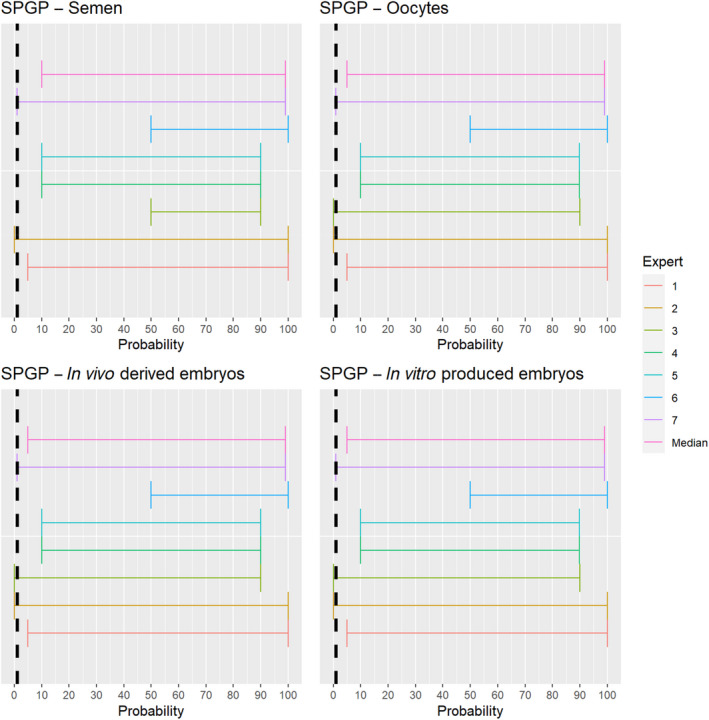
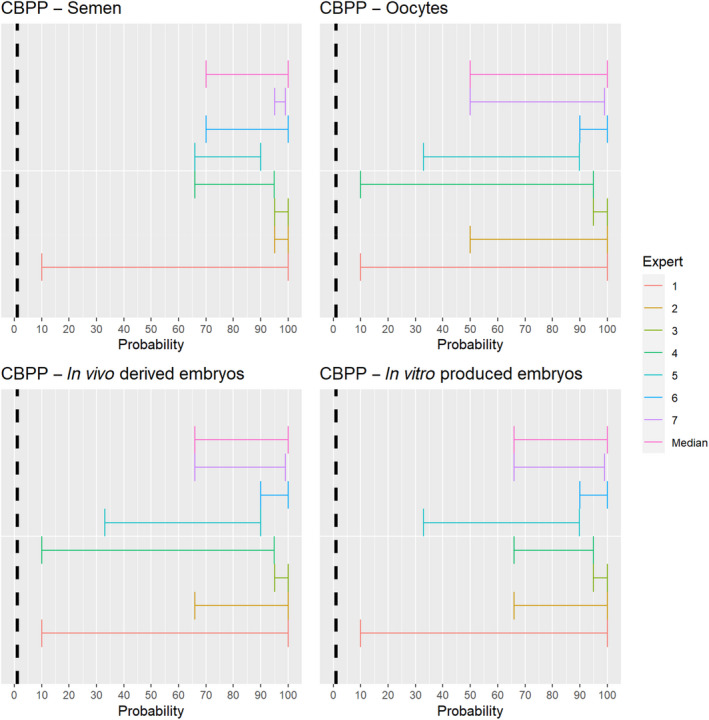
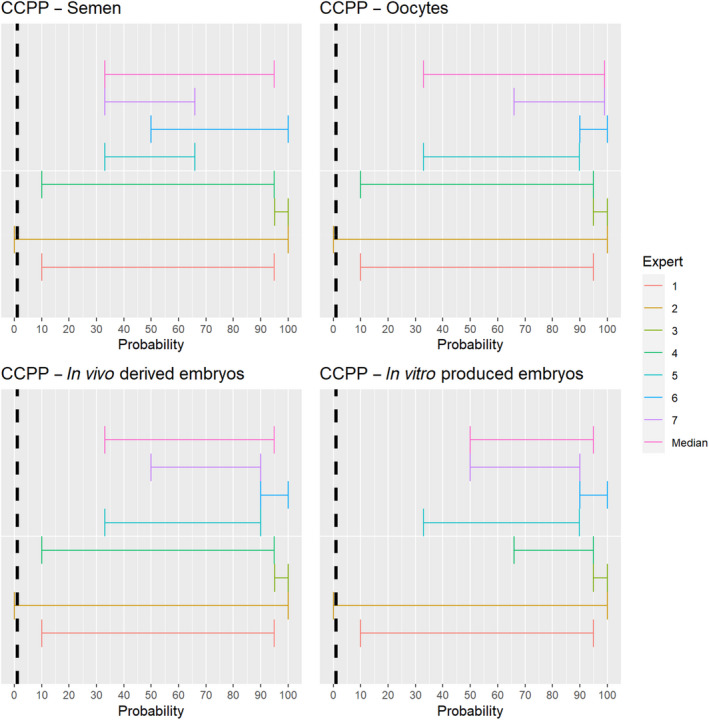
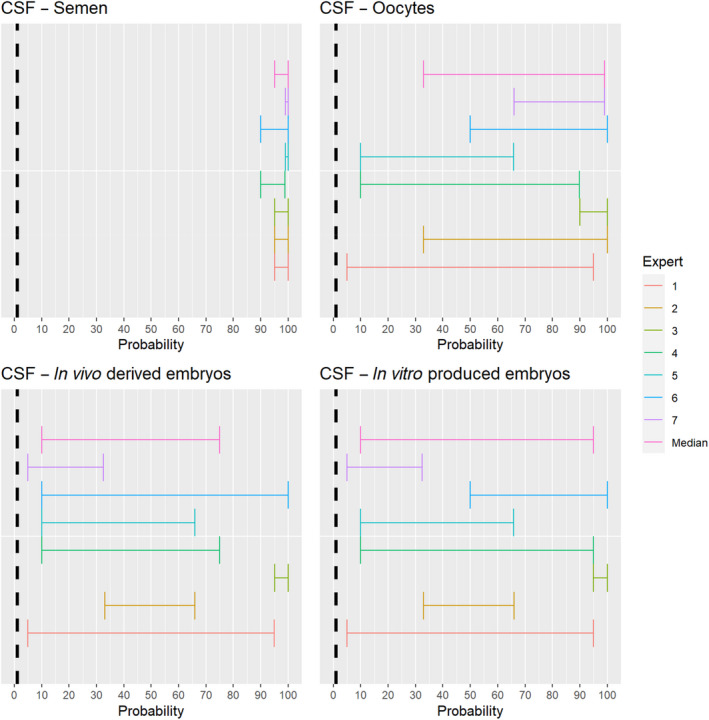
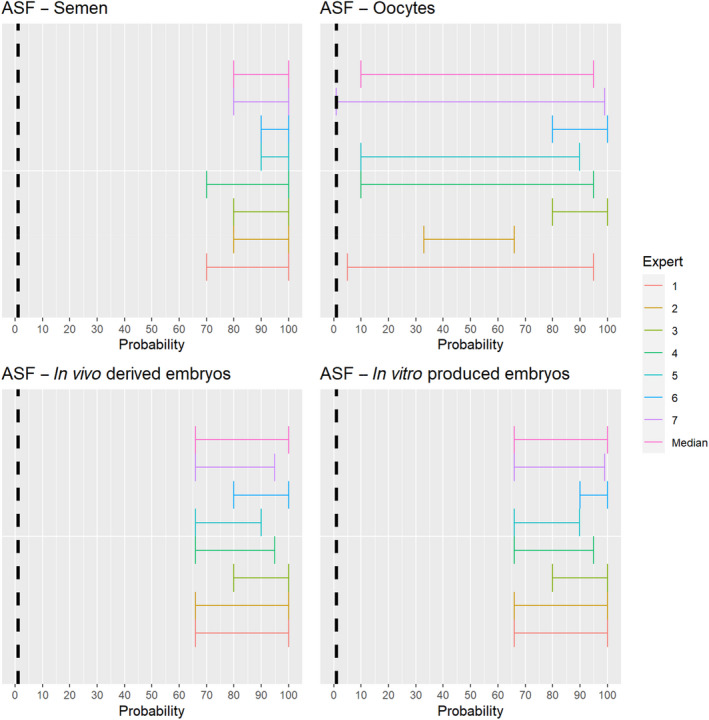
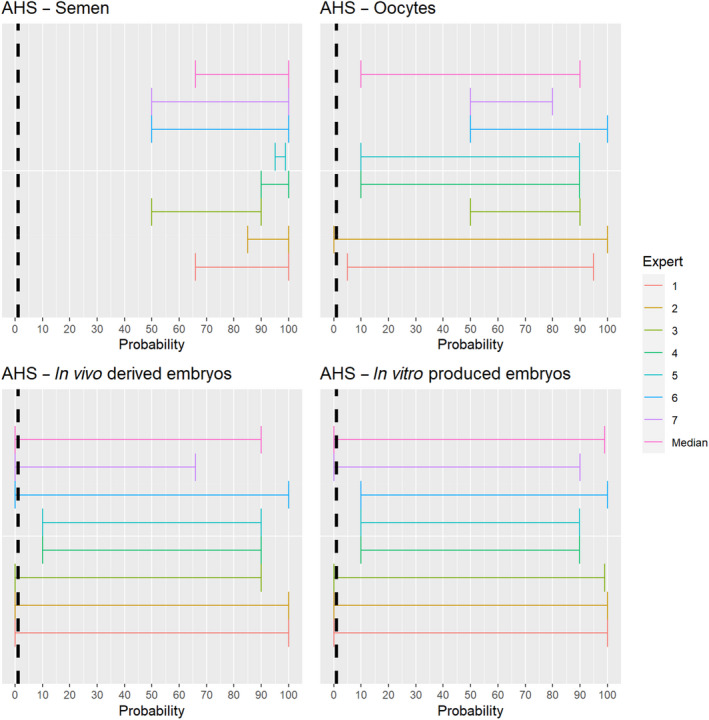
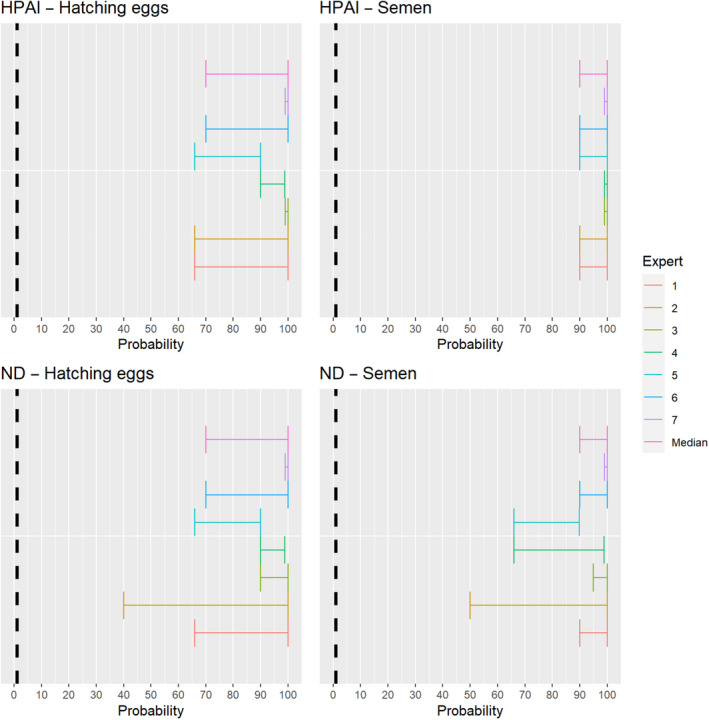
A.3. Final individual judgements of the assessment of prohibitions of movements of animal products, animal by‐products and movements of feed of plant origin and straw
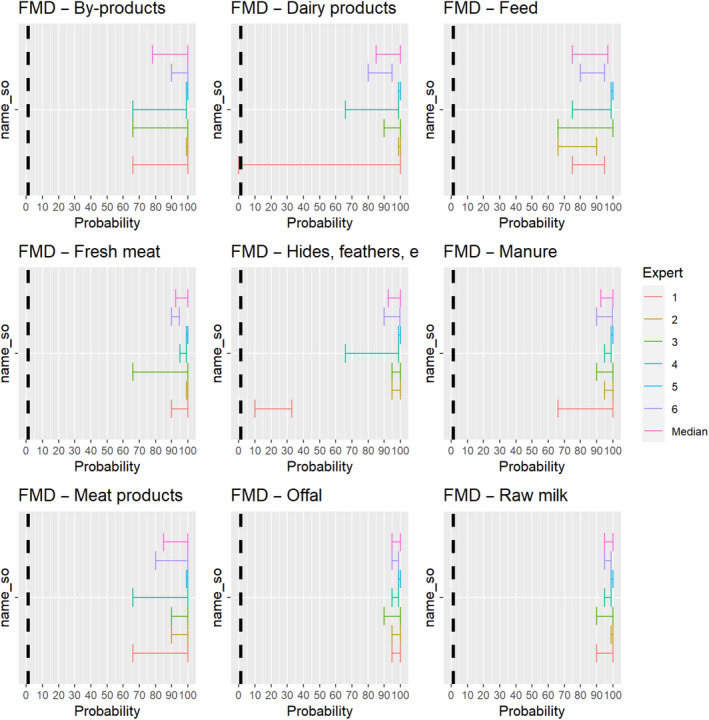
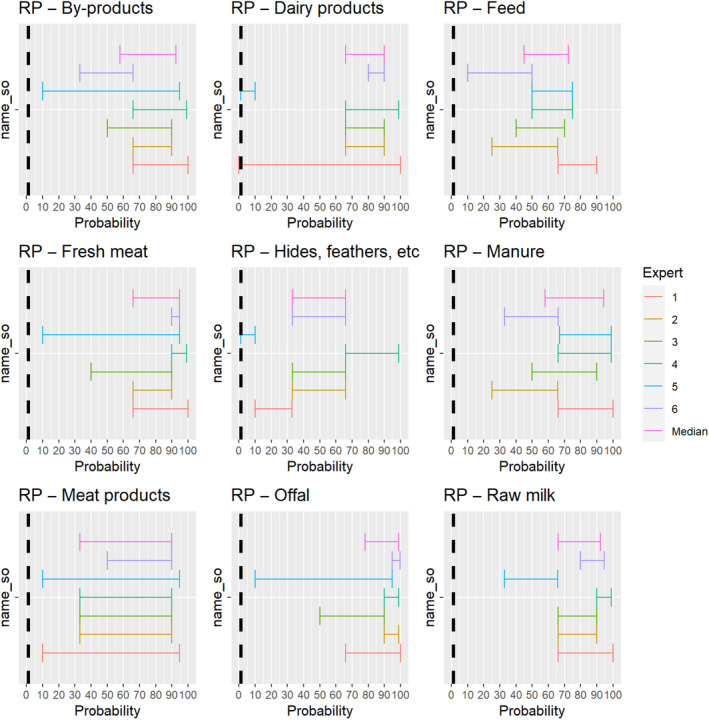
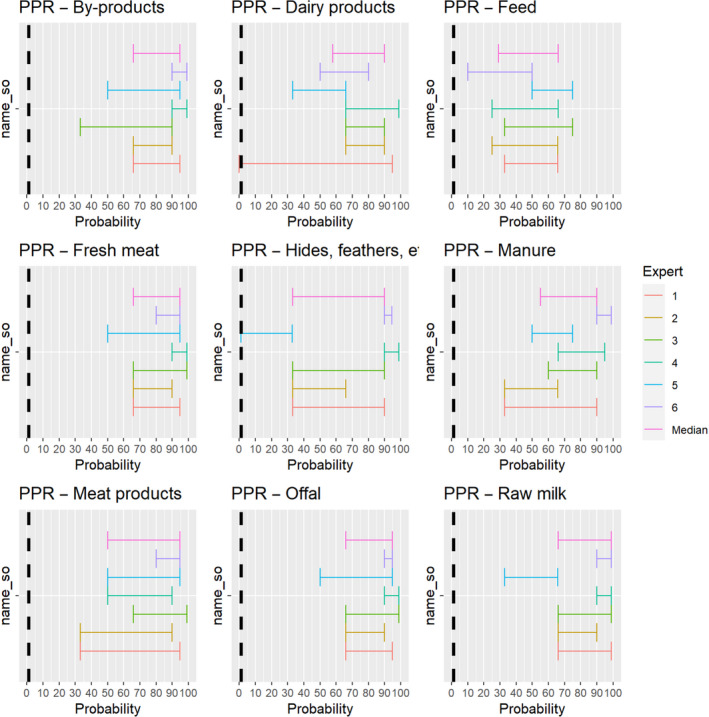
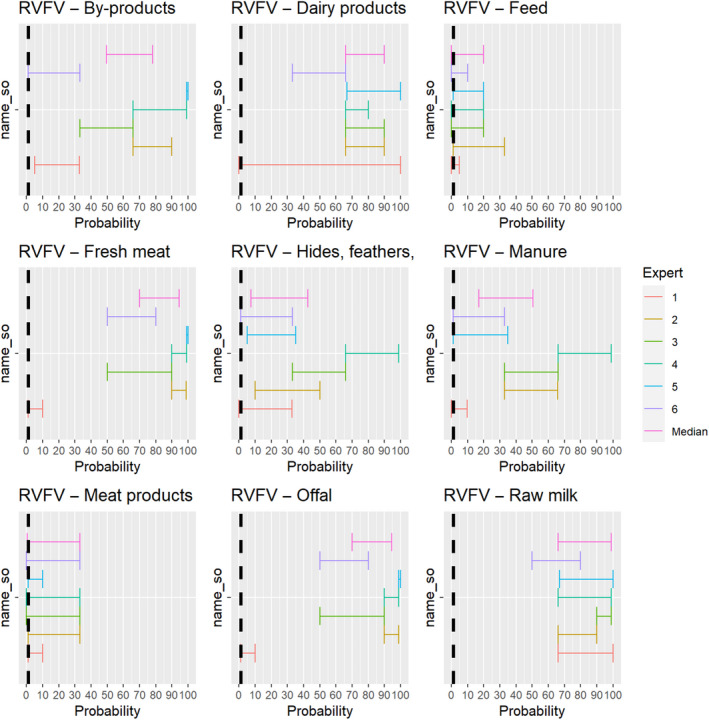
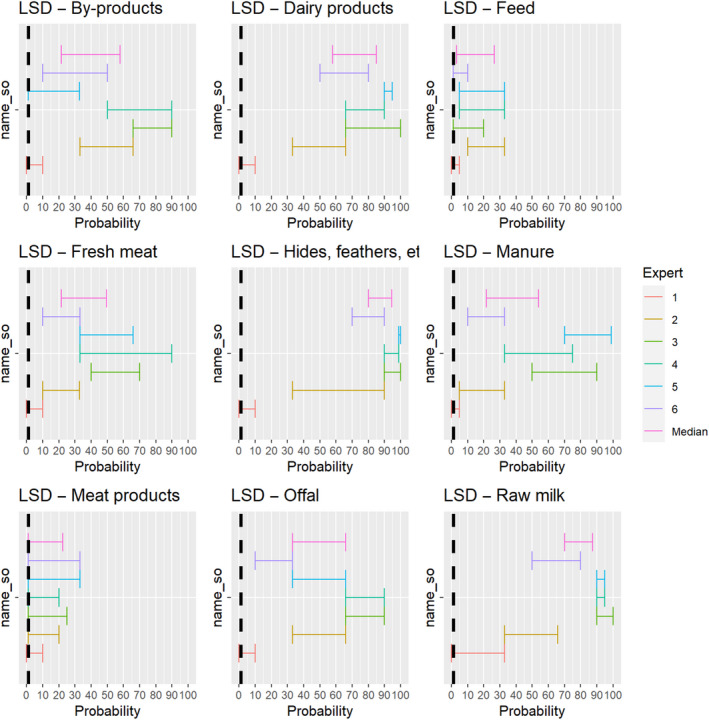
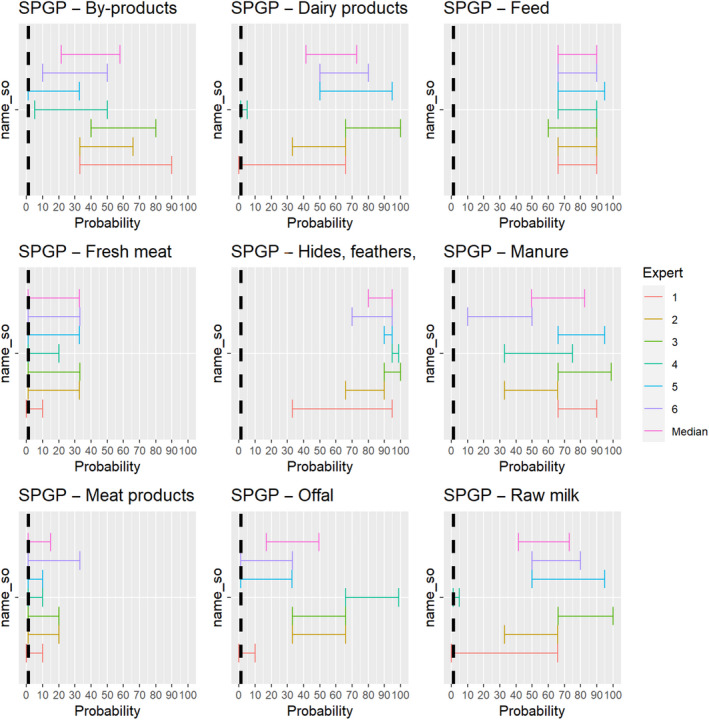
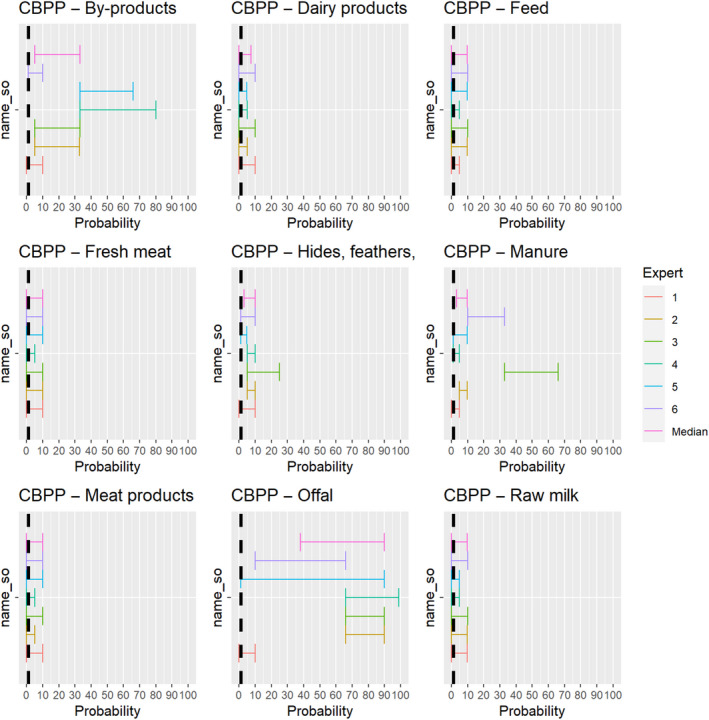
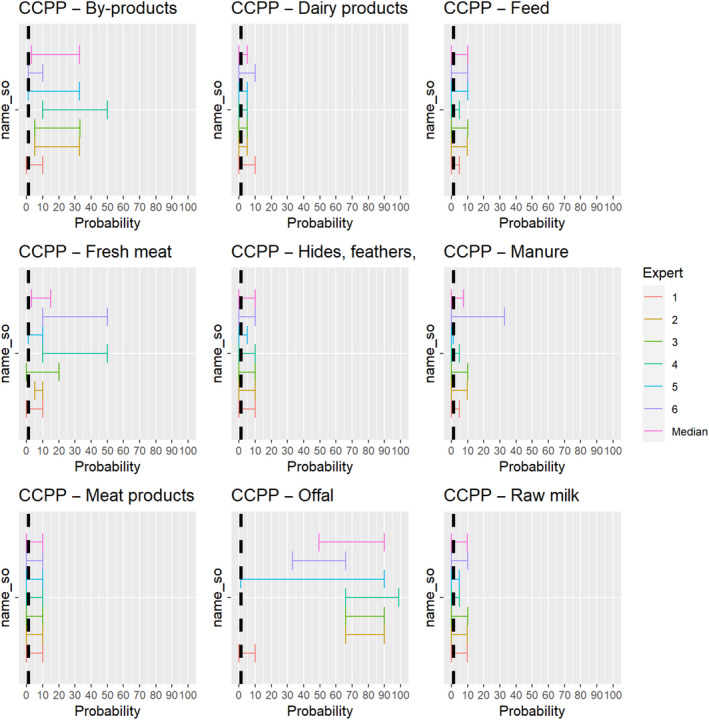
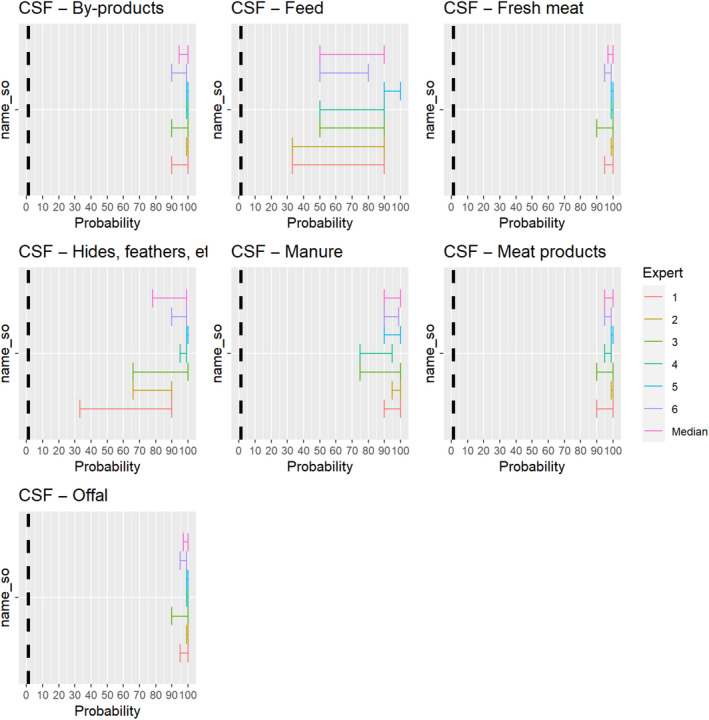
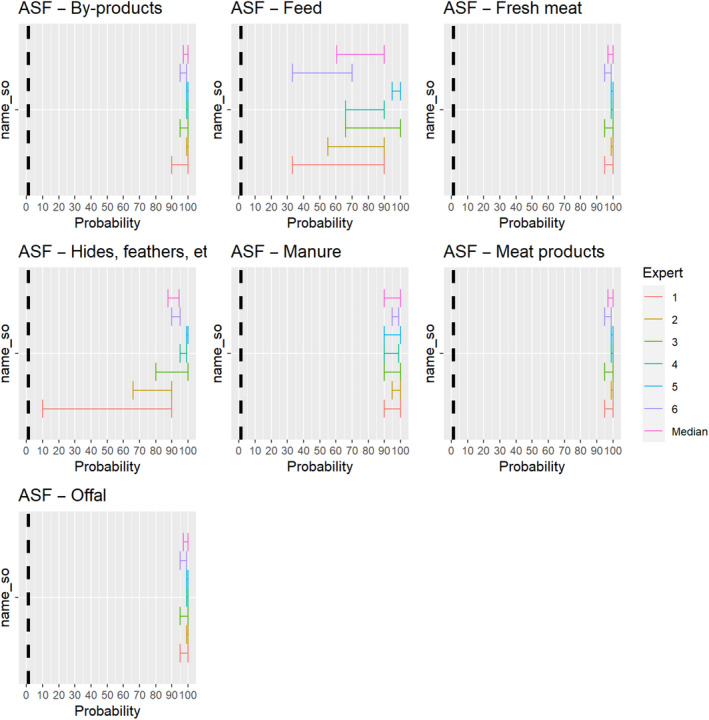
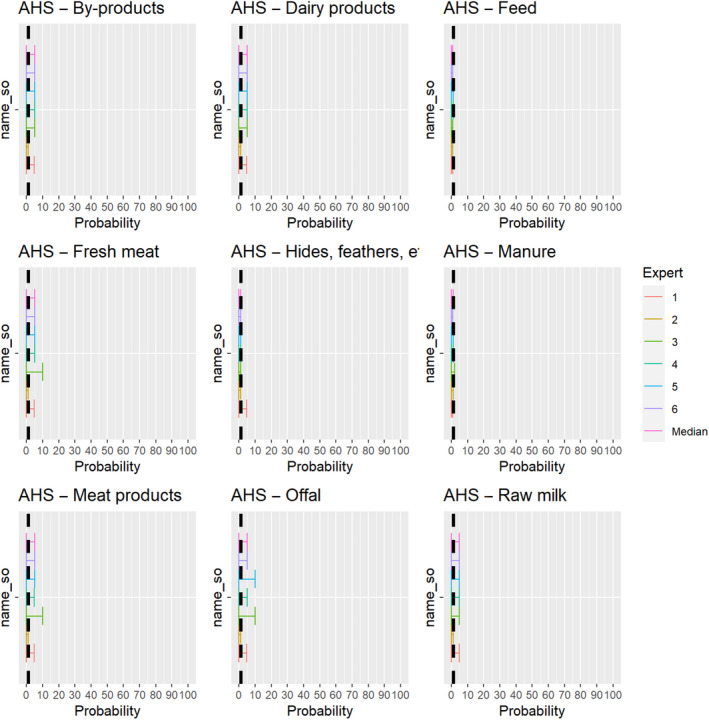
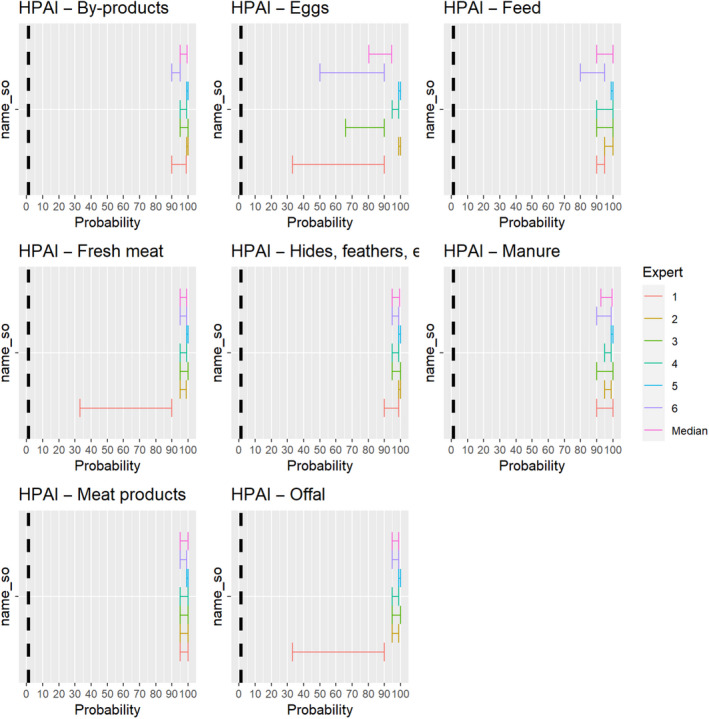
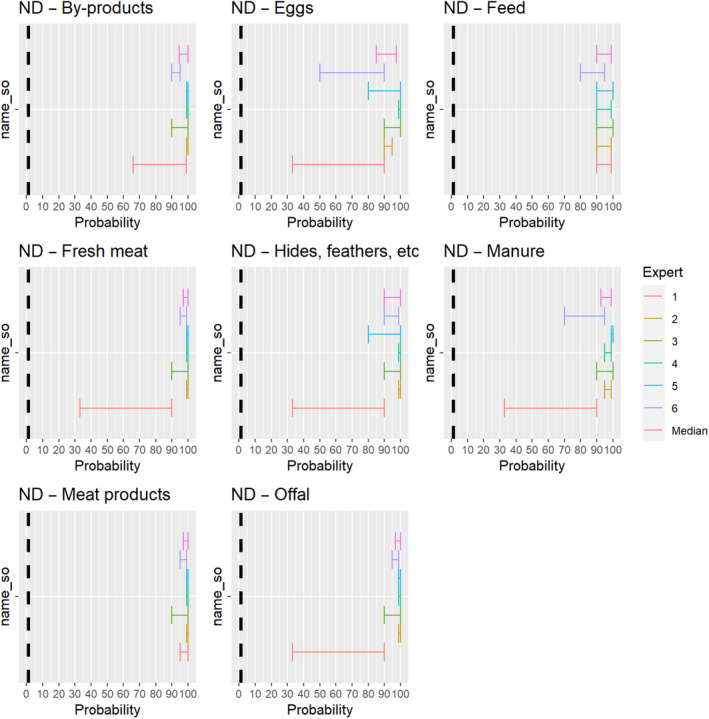
A.4. Final individual judgements of the assessment of risk mitigation treatments
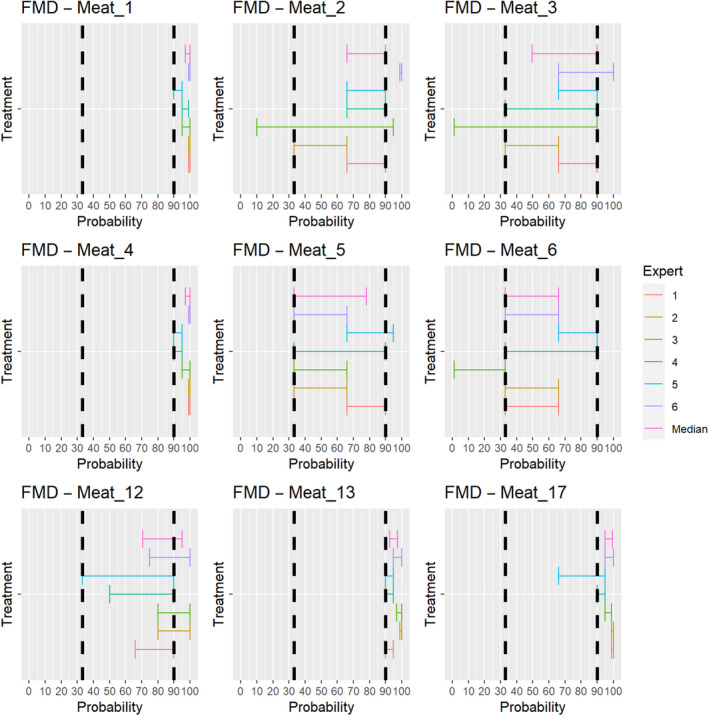
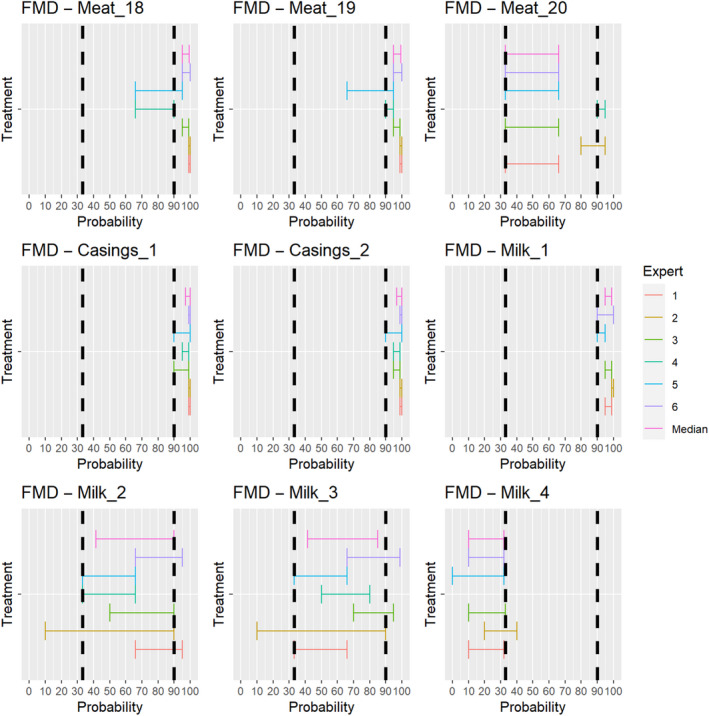
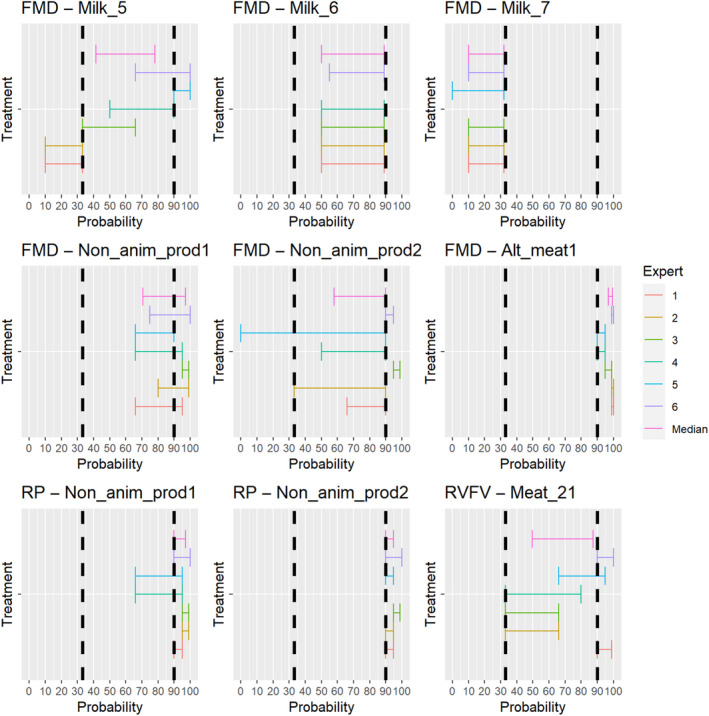
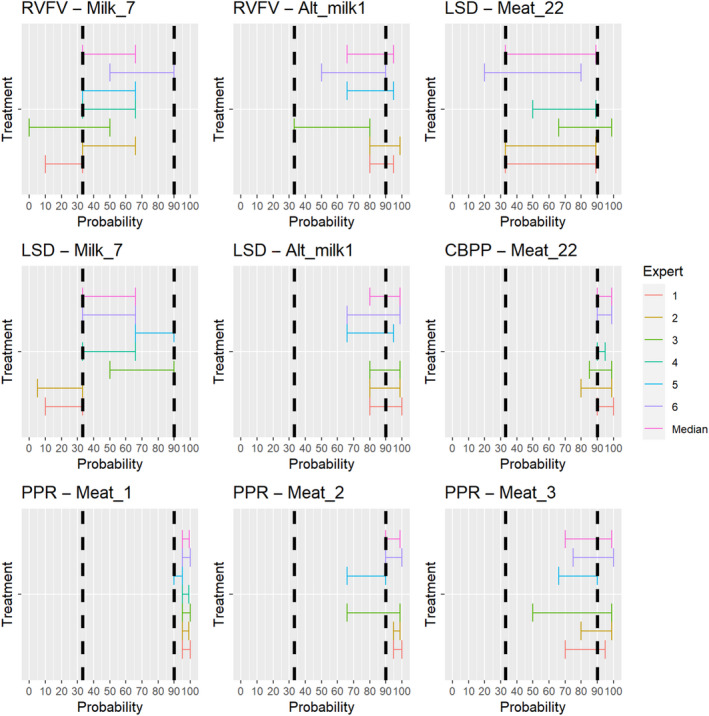
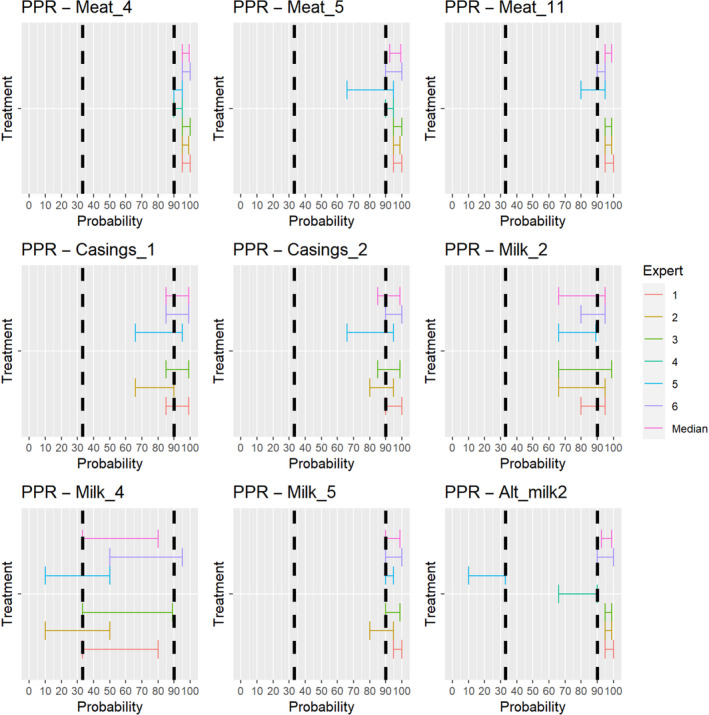
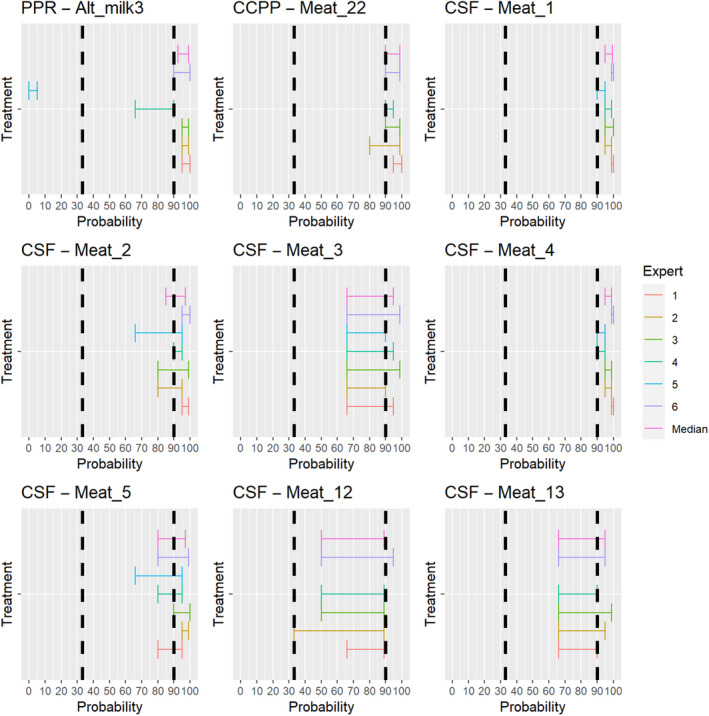
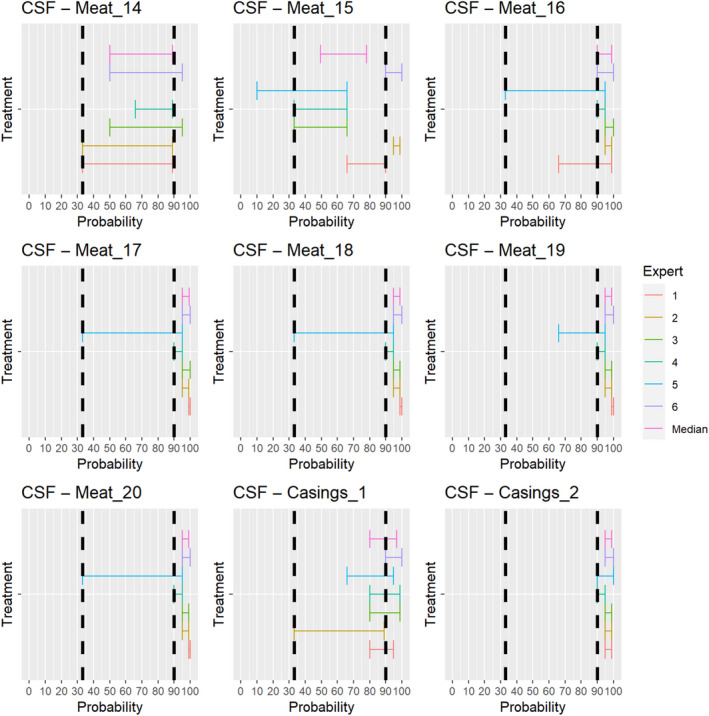
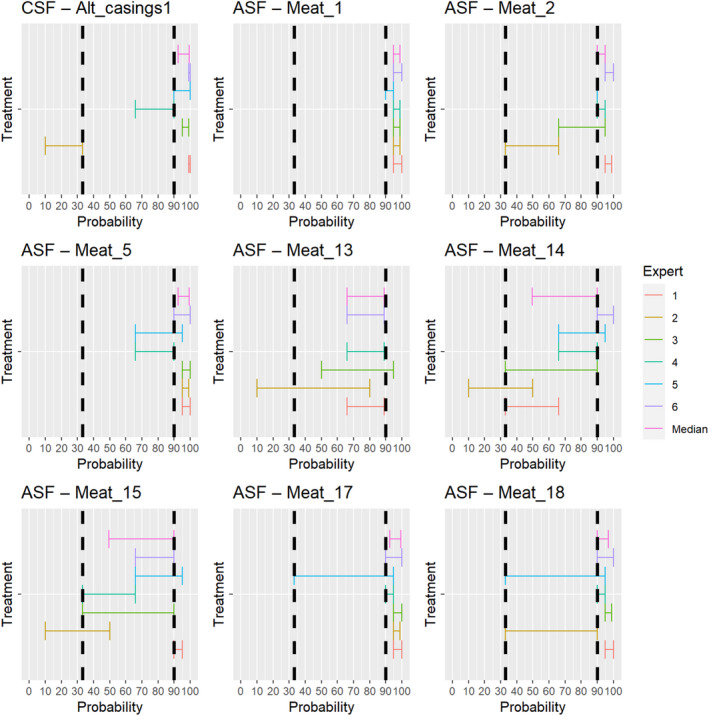
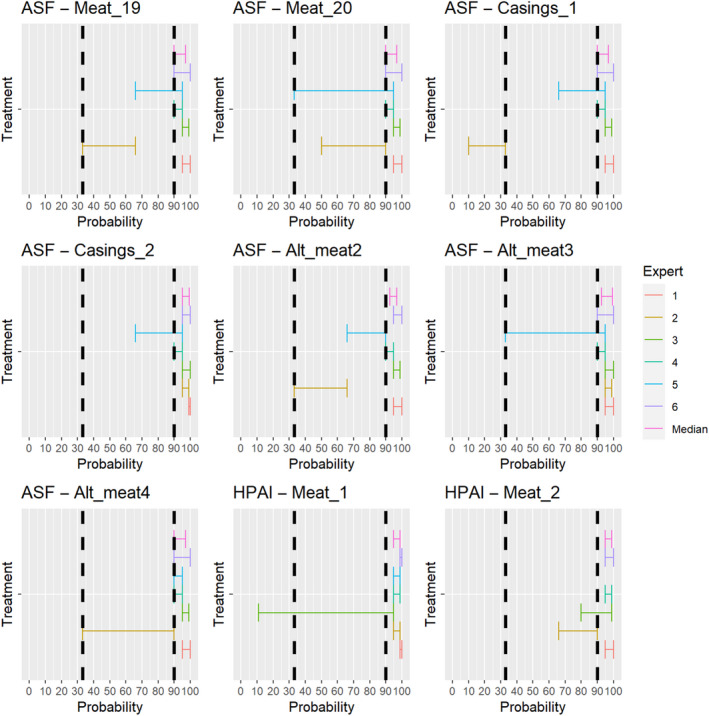
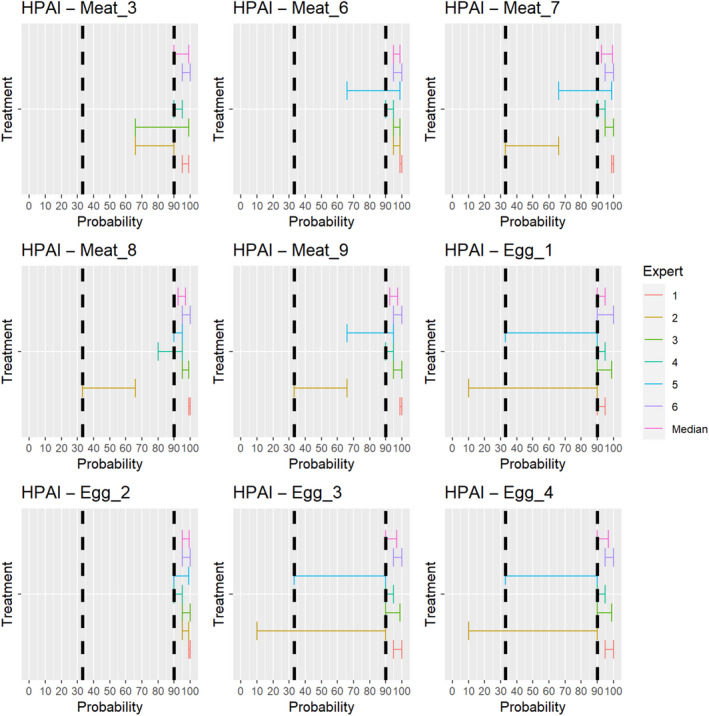
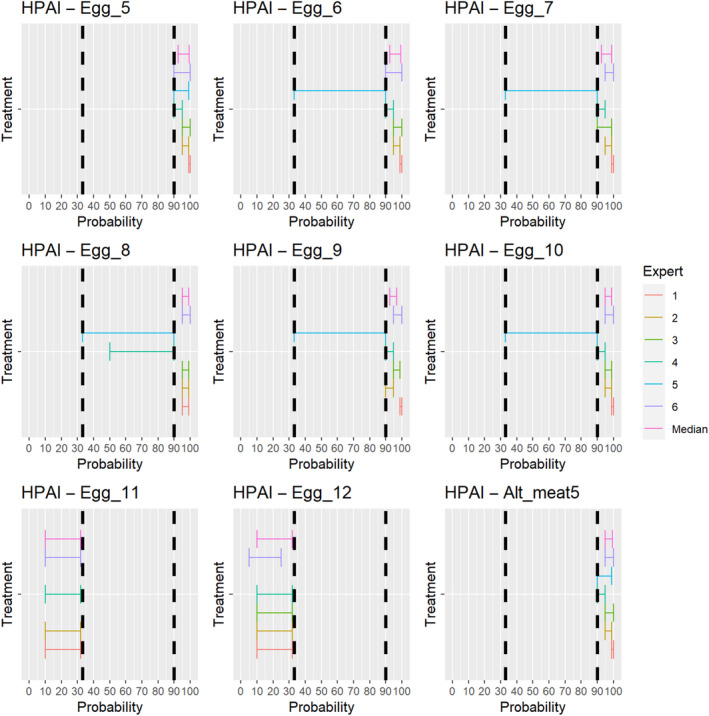
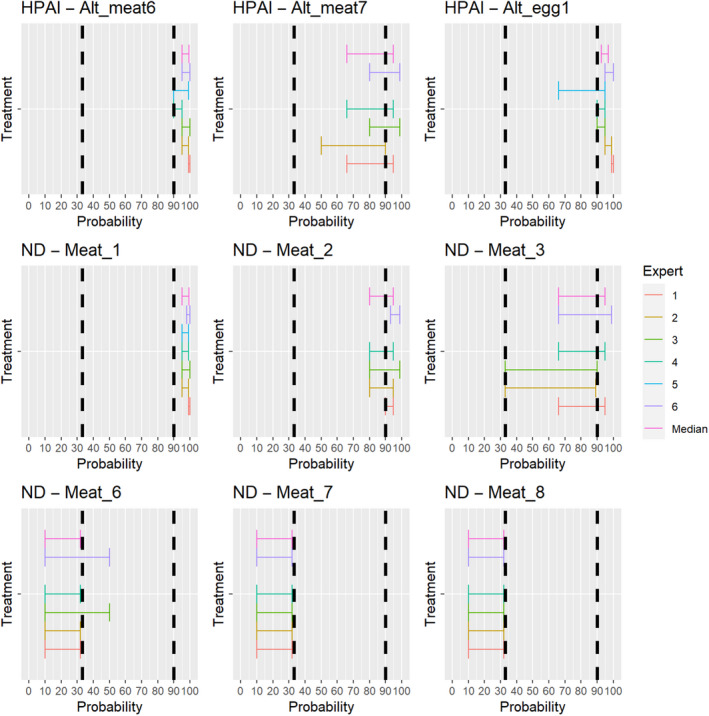
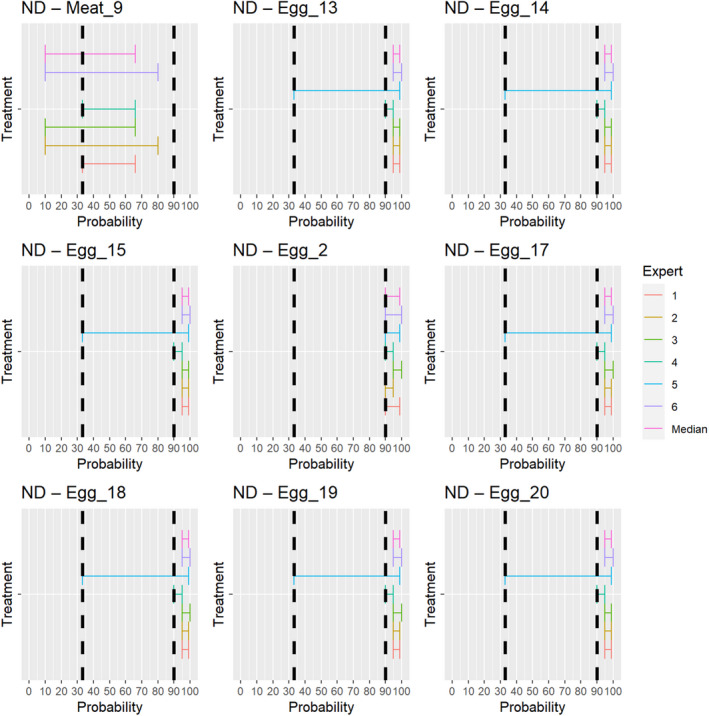
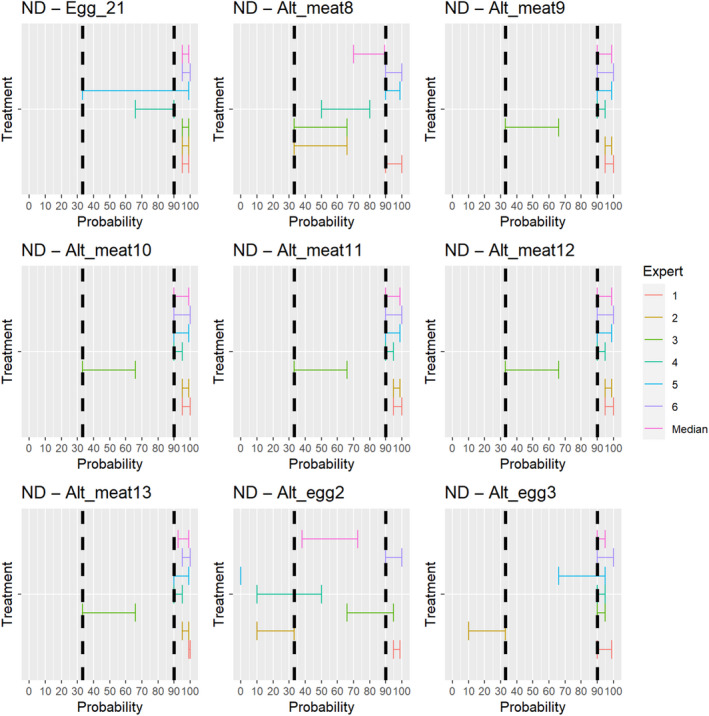
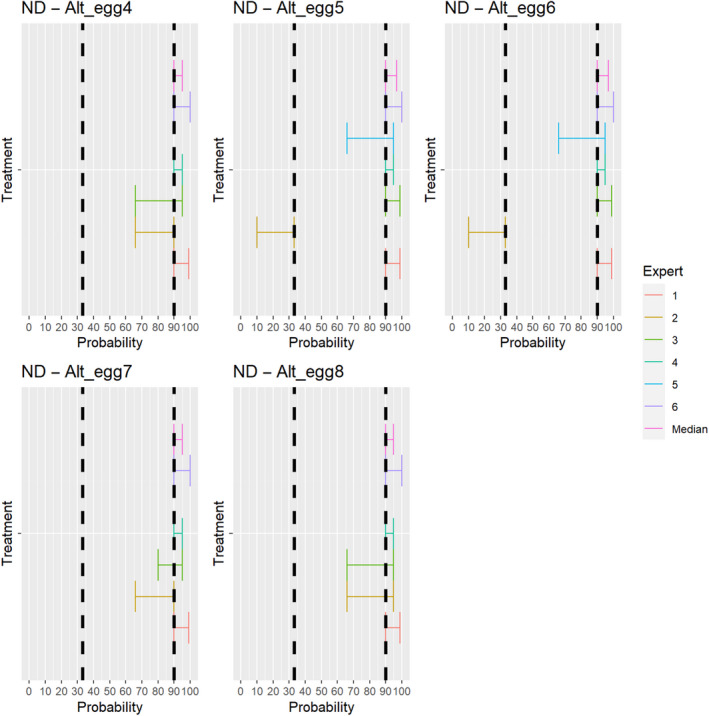
Appendix B – Risk mitigation treatments for which recommendations are made
B.1. Treatments assessed as inconclusive regarding mitigation of the risk of spreading the Category A diseases due to incomplete treatment description
Meat
| Treatment full name | Disease | |
|---|---|---|
| Meat_2 | Meat heat‐treated to achieve a core of temperature of 80°C | FMD, CSF, ND |
| Meat_3 | Meat heat‐treated to achieve a core of temperature of 70°C | FMD, PPR, CSF, ND |
Milk
| Treatment full name | Disease | |
|---|---|---|
| Milk_3 | Milk heat treatment UHT (Ultra‐high temperature): Minimum 135°C for a suitable holding time | FMD |
B.2. Treatments assessed as inconclusive regarding mitigation of the risk of spreading the Category A diseases due to insufficient/inconclusive scientific evidence
Meat
| Treatment full name | Disease | |
|---|---|---|
| Meat_5 | Meat treated in a hermetically sealed container, applying 60°C for a minimum of 4 h | FMD, CSF |
| Meat_6 | Meat heated to a core temperature of 73.9°C for a minimum of 0.51 s | FMD |
| Meat_9 | Meat core temperature of 60.0°C for a minimum of 507 s | ND |
| Meat_12 | Natural fermentation and maturation for bone‐in meat: minimum 9 months, to achieve maximum values of Aw of 0.93 and pH of 6 | FMD, CSF |
| Meat_13 | Natural fermentation and maturation for de‐boned meat: minimum 9 months, to achieve maximum values of Aw of 0.93 and pH of 6 | CSF, ASF |
| Meat_14 | Natural fermentation for loins: minimum 140 days to achieve maximum values of Aw of 0.93 and pH of 6 | CSF, ASF |
| Meat_15 | Natural fermentation for hams: minimum 190 days to achieve maximum values of Aw of 0.93 and pH of 66 | CSF, ASF |
| Meat_20 | Drying after salting Serrano hams: minimum 140 days | FMD |
| Meat_22 | Removal of offal | LSD |
| Meat_temp_21 | Maturation of carcasses at a minimum temperature of 2°C for a minimum of 24 h following slaughter | RVF |
| Alt_meat7 | Incubation at 500 MPa at 15 °C for a minimum of 15 s | HPAI |
| Alt_meat8 | Meat core temperature of 65.0°C for a minimum of 120 s | ND |
Casings
| Treatment full name | Disease | |
|---|---|---|
| Casings_1 | Casing salting with sodium chloride (NaCl) either dry or as saturated brine (Aw < 0.80), for a continuous period of 30 days or longer at an ambient temperature of 20°C or above | PPR, CSF |
| Casings_2 | Casing salting with phosphate supplemented salt 86.5% NaCl, 10.7% Na2HPO4 and 2.8% Na3PO4 either dry or as saturated brine (Aw < 0.80) for a continuous period of 30 days or longer at an ambient temperature of 20°C or above | PPR |
Milk
| Treatment full name | Disease | |
|---|---|---|
| Milk_2 | Milk heat treatment UHT (Ultra‐high temperature): Minimum 132°C for a minimum of 1 s | FMD, PPR |
| Milk_4 | Milk heat treatment HTST (High‐temperature short‐time) pasteurisation if milk pH is lower than 7, minimum 72°C for a minimum of 15 s | PPR |
| Milk_5 | Milk heat treatment HTST (High‐temperature short‐time) pasteurisation if milk pH is 7 or higher, minimum 72°C for a minimum of 15 s, applied twice | FMD |
| Milk_6 | Milk heat treatment HTST (High‐temperature short‐time) pasteurisation combined with a physical treatment to achieve pH value below 6 for a minimum of 1 h or HTST pasteurisation to achieve a minimum of 72°C, combined with desiccation | FMD |
| Milk_7 | Milk pasteurisation consisting in a single heat treatment with an effect at least equivalent to that achieved by applying 72°C for 15 s | RVF, LSD |
| Alt_milk1 | Milk pasteurisation consisting in a single heat treatment with an effect at least equivalent to that achieved by applying 72°C for 15 s with additional acidification | RVF, LSD |
B.3. Treatments assessed as not effective to mitigate the risk of spreading the Category A diseases
Meat
| Treatment full name | Disease | |
|---|---|---|
| Meat_6 | Meat core temperature of 73.9°C for a minimum of 0.51 s | NDV |
| Meat_7 | Meat core temperature of 70.0°C for a minimum of 3.5 s | NDV |
| Meat_8 | Meat core temperature of 65.0°C for a minimum of 42 s | NDV |
Milk
| Treatment full name | Disease | |
|---|---|---|
| Milk_4 | Milk heat treatment HTST (High‐temperature short‐time) pasteurisation if milk pH is lower than 7, minimum 72°C for a minimum of 15 s | FMD |
| Milk_7 | Milk pasteurisation consisting in a single heat treatment with an effect at least equivalent to that achieved by applying 72°C for 15 s | FMD |
Eggs
| Treatment full name | Disease | |
|---|---|---|
| Egg_11 | Dried egg white: 54.4°C ‐ 50.4 h | HPAI |
| Egg_12 | Dried egg white: 51.7°C ‐ 73.2 h | HPAI |
Annex A – Extensive literature search on the presence of selected Category A disease pathogens in germinal products
Annex A can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7443
Annex B – Extensive literature search on the presence of selected Category A disease pathogens in animal products, animal by‐products and feed of plant origin and straw
Annex B can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7443
Annex C – Extensive literature search on the effectiveness of risk mitigation treatments for products of animal origin and products of non‐animal origin
Annex C can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7443
Abbreviations
- AHS
African horse sickness
- AHSV
African horse sickness virus
- ASF
African swine fever
- ASFV
African swine fever virus
- CBPP
contagious bovine pleuropneumonia
- CCPP
contagious caprine pleuropneumonia
- CSF
classical swine fever
- CSFV
classical swine fever virus
- DR
Delegated Regulation
- ELS
extensive literature Search
- FMD
foot and mouth disease
- FMDV
foot and mouth disease virus
- HPAI
highly pathogenic avian influenza
- HTST
high‐temperature short‐time pasteurisation
- LSD
lumpy skin disease
- LSDV
lumpy skin disease virus
- ND
Newcastle disease
- NDV
Newcastle disease virus
- PPR
Peste des petit ruminants
- PPRV
Peste des petit ruminants virus
- RP
Rinderpest
- RPV
Rinderpest virus
- RVF
Rift Valley fever
- RVFV
Rift Valley fever virus
- SPGP
sheep and goat pox
- SPGPV
sheep and goat pox virus
- ToR
Term of Reference
- UHT
ultra‐high temperature
- WG
working group
- WOAH
World Organisation for Animal Health
Supporting information
Extensive literature search on the presence of selected Category A disease pathogens in germinal products
Extensive literature search on the presence of selected Category A disease pathogens in animal products, animal by‐products and feed of plant origin and straw
Extensive literature search on the effectiveness of risk mitigation treatments for products of animal origin and products of non‐animal origin
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Schmidt CG, Herskin M, Michel V, Miranda Chueca MA, Padalino B, Pasquali P, Stahl K, Calvo AV, Viltrop A, Winckler C, De Clercq K, Sjunnesson Y, Gervelmeyer A and Roberts HC, 2022. Scientific Opinion on the assessment of the control measures of the Category A diseases of the Animal Health Law: prohibitions in restricted zones and risk‐mitigating treatments for products of animal origin and other materials. EFSA Journal 2022;20(8):7443, 128 pp. 10.2903/j.efsa.2022.7443
Requestor: European Commission
Question number: EFSA‐Q‐2022‐00024
Panel members: Søren Saxmose Nielsen, Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Elisabetta Canali, Julian Ashley Drewe, Bruno GarinBastuji, José Luis Gonzales Rojas, Christian Gortázar Schmidt, Mette Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Barbara Padalino, Paolo Pasquali, Helen Clare Roberts, Karl Stahl, Antonio Velarde Calvo, Arvo Viltrop and Christoph Winckler.
Declarations of interest: If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgments: The Panel wishes to thank the following for the support provided to this scientific output: Verena Oswaldi, Inmaculada Aznar Asensio from EFSA.
Adopted: 30 June 2022
Notes
Questions answered by the experts: Can infected animals of listed species transmit the pathogen as a result of the activity? Can the germinal product collected from infected animals of listed species contain the infectious pathogen? Can the product collected from infected animals of listed species (including game and wild animals, where mentioned in Annex VI) transmit the pathogen as a result of the movement?/ Can the pathogen be transmitted via the contaminated material as a result of the movement?
Questions answered by the experts: Is this treatment effective in inactivating the pathogen if it is present in this product or material?
Heat treatment, minimum temperature of 80°C and for a minimum of 10 min, steam in a closed chamber.
Storage in package or bales under shelter at premises situated not closer than 2 km to the nearest outbreak and releasing from the premises do not take place before at least three months have elapsed following the completion of cleaning and disinfection according to Article 15.
References
- Alexander DJ and Manvell RJ, 2004. Heat inactivation of Newcastle disease virus (strain Herts 33/56) in artificially infected chicken meat homogenate. Avian Pathology, 33, 222–225. 10.1080/0307945042000195795 [DOI] [PubMed] [Google Scholar]
- Annandale CH, Holm DE, Ebersohn K and Venter EH, 2014. Seminal transmission of lumpy skin disease virus in heifers. Transboundary and Emerging Diseases, 61, 443–448. 10.1111/tbed.12045 [DOI] [PubMed] [Google Scholar]
- Bielanski A, 2014. Biosafety in embryos and semen cryopreservation, storage, management and transport. Advances in Experimental Medicine and Biology, 753, 429–465. 10.1007/978-1-4939-0820-2_17 [DOI] [PubMed] [Google Scholar]
- Blackwell JH and Hyde JL, 1976. Effect of heat on foot‐and‐mouth disease virus (FMDV) in the components of milk from FMDV‐infected cows. Journal of Hygiene (London), 77, 77–83. 10.1017/s0022172400055534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona C, Wileman B, Malladi S, Ceballos R, Culhane M, Munoz‐Aguayo J, Flores‐Figueroa C, Halvorson D, Walz E, St. Charles K and Bonney P, 2021. The risk of highly pathogenic influenza A virus transmission to turkey hen flocks through artificial insemination. Avian Diseases, 65, 303–309. 10.1637/aviandiseases-D-20-00132 [DOI] [PubMed] [Google Scholar]
- CFSPH (Center for food security and public health) , 2017. Sheep pox and goat pox. Available online: https://www.cfsph.iastate.edu/Factsheets/pdfs/sheep_and_goat_pox.pdf
- Dhama K, Singh RP, Karthik K, Chakraborty S, Tiwari R, Wani MY and Mohan J, 2014. Artificial insemination in poultry and possible transmission of infectious pathogens: a review. Asian Journal of Animal and Veterinary Advances, 9, 211–228. 10.3923/ajava.2014.211.228 [DOI] [Google Scholar]
- Dhennin L and Labie J, 1976. Thermorésistance du virus de la fièvre aphteuse dans le lait de vaches infectées. Bulletin de l'Académie Vétérinaire de France, 49, 243–249. 10.4267/2042/65941 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2006. Opinion on the Animal health risks of feeding animals with ready to use dairy products without further treatment. EFSA Journal 2006;4: 347. 10.2903/j.efsa.2006.347 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Boklund A, Bøtner A, Chesnoiu Vasile T, Depner K, Desmecht D, Guberti V, Helyes G, Korytarova D, Linden A, Miteva A, More S, Olsevskis E, Ostojic S, Roberts H, Spiridon M, Stahl K, Thulke HH, Vilija G, Viltrop A, Wallo R, Wozniakowski G, Abrahantes Cortinas J, Dhollander, Gogin A , Ivanciu C, Papanikolaou A, Villeta Gonzalez LC and Gortazar Schmidt C, 2020. Scientific report on the epidemiological analyses of African swine fever in the European Union (November 2018 to October 2019). EFSA Journal 2020;18(1):5996, 107 pp. 10.2903/j.efsa.2021.5996 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Depner K, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MÁ, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, De Clercq K, Klement E, Stegeman JA, Gubbins S, Antoniou S‐E, Broglia A, Van der Stede Y, Zancanaro G and Aznar I, 2021a. Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: African Horse Sickness. EFSA Journal 2021;19(2):6403, 70 pp. 10.2903/j.efsa.2021.6403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Depner K, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MÁ, Pasquali P, Roberts, HC , Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, De Clercq K, Klement E, Stegeman JA, Gubbins S, Antoniou S‐E, Broglia A, Van der Stede Y, Zancanaro G and Aznar I, 2021b. Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: African Swine Fever. EFSA Journal 2021;19(1):6402, 82 pp. 10.2903/j.efsa.2021.6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Gubbins S, Stegeman JA, Antoniou SE, Aznar I, Broglia A, Lima E, Van der Stede Y , Zancanaro G and Roberts HC, 2021c. Assessment of the control measures of the category A diseases of Animal Health Law: Classical Swine Fever. EFSA Journal 2021;19(7):6707, 83 pp. 10.2903/j.efsa.2021.6707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, De Clercq K, Gubbins, S , Klement E, Stegeman JA, Antoniou S‐E, Aznar I, Broglia A, Papanikolaou A, Van der Stede Y, Zancanaro G and Roberts HC, 2021d. Scientific Opinion on the assessment of the control measures for category A diseases of Animal Health Law: Foot and Mouth Disease. EFSA Journal 2021;19(6):6632, 85 pp. 10.2903/j.efsa.2021.6632 [DOI] [PMC free article] [PubMed]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Depner K, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Schmidt CG, Herskin M, Michel V, Miranda Chueca MÁ, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Stahl K, Calvo AV, Viltrop A, Winckler C, De Clercq K, Klement E, Stegeman JA, Gubbins S, Antoniou S‐E, Broglia A, Van der Stede Y, Zancanaro G and Aznar I, 2021e. Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: Highly Pathogenic Avian Influenza. EFSA Journal 2021;19(1):6372, 78 pp. 10.2903/j.efsa.2021.6372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Gubbins S, Stegeman JA, Antoniou S‐E, Aznar I, Broglia A, Van der Stede Y, Zancanaro G and Roberts HC, 2021f. Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: Newcastle disease. EFSA Journal 2021;19(12):6946, 85 pp. 10.2903/j.efsa.2021.6946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Depner K, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino, B , Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Gubbins S, Libeau G, Broglia A, Aznar I and Van der Stede, Y , 2021g. Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: peste des petits ruminants. EFSA Journal 2021;19(7):6708, 94 pp. 10.2903/j.efsa.2021.6708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, De Clercq K, Gubbins S, Aznar I and Broglia A, 2021h. Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: sheep and goat pox. EFSA Journal 2021;19(12):6933, 89 pp. 10.2903/j.efsa.2021.6933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Schmidt CG, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Gubbins S, Laroucau K, Antoniou S‐E, Aznar I, Broglia A, Lima E, Van der Stede Y, Zancanaro G and Roberts HC, 2022a. Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: Burkholderia mallei (Glanders). EFSA Journal 2022;20(1):7069, 60 pp. 10.2903/j.efsa.2022.7069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Gubbins S, Stegeman JA, Thiaucourt F, Antoniou S‐E, Aznar I, Papanikolaou A, Zancanaro G and Roberts HC, 2022b. Scientific Opinion on the assessment of the control measures for category A diseases of Animal Health Law: Contagious Caprine Pleuropneumonia. EFSA Journal 2022;20(1):7068, 88 pp. 10.2903/j.efsa.2022.7068 [DOI] [PMC free article] [PubMed]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, Gubbins S, Stegeman JA, Thiaucourt F, Antoniou S‐E, Aznar I, Papanikolaou A, Zancanaro G and Roberts HC, 2022c. Scientific Opinion on the assessment of the control measures for category A diseases of Animal Health Law: Contagious Bovine Pleuropneumonia. EFSA Journal 2022;20(1):7067, 96 pp. 10.2903/j.efsa.2022.7067 [DOI] [PMC free article] [PubMed]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar Schmidt C, Herskin M, Michel V, Miranda Chueca MÁ, Padalino B, Pasquali P, Sihvonen LH, Spoolder H, Ståhl K, Velarde A, Viltrop A, Winckler C, De Clercq K, Gubbins S, Klement E, Stegeman JA, Antoniou S‐E, Aznar I, Broglia A, Van der Stede Y, Zancanaro G and Roberts HC, 2022d. Scientific Opinion on the assessment of the control measures for category A diseases of Animal Health Law: Lumpy Skin Disease. EFSA Journal 2022;20(1):7121, 70 pp. 10.2903/j.efsa.2022.7121 [DOI] [PMC free article] [PubMed]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Canali E, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortázar C, Herskin M, Michel V, Miranda Chueca MÁ, Roberts HC, Padalino B, Pasquali P, Spoolder H, Ståhl K, Calvo AV, Viltrop A, Winckler C, Gubbins S, Broglia A, Aznar I and Van der Stede Y, 2022e. Scientific Opinion on the assessment of the control measures of the category A diseases of Animal Health Law: Rift Valley Fever. EFSA Journal 2022; 20(1):7070, 85 pp. 10.2903/j.efsa.2022.7070 [DOI] [PMC free article] [PubMed]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen, SS , Alvarez, J , Bicout, DJ , Calistri, P , Canali, E , Drewe, JA , Garin‐Bastuji, B , Gonzales Rojas, JL , Gortázar, C , Herskin, M , Michel, V , Miranda Chueca, MÁ , Padalino, B , Pasquali, P , Spoolder, H , Ståhl, K , Velarde, A , Viltrop, A , Winckler, C , De Clercq, K , Gubbins, S , Libeau, G , Gervelmeyer, A and Roberts, HC , 2022f. Scientific Opinion on the assessment of the control measures of category A diseases of the Animal Health Law: Infection with rinderpest virus (Rinderpest). EFSA Journal 2022;20(1):7071, 79 pp. 10.2903/j.efsa.2022.7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Germini A, Martino L, Merten C, Mosbach‐Schulz O, Smith A and Hardy A, 2018. Scientific Opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed]
- Ellin DM, 2010. White Paper on Effectiveness of Existing Interventions on Virus Inactivation in Meat and Poultry Products. Food Research Institute, University of Wisconsin Food. Available online: https://meatpoultryfoundation.org/namif/wp-content/uploads/08-404.pdf [Google Scholar]
- ILSI , 2009. Europe Expert Group on Animal Borne Viruses. Animal‐borne viruses of relevance to the food industry. Brussels, Belgium, ILSI Europe a.i.s.b.l. Available online: https://ilsi.eu/publication/animal-borne-viruses-of-relevance-to-the-food-industry/ [Google Scholar]
- Isbarn S, Buckow R, Himmelreich A, Lehmacher A and Heinz V, 2007. Inactivation of avian influenza virus by heat and high hydrostatic pressure. Journal of Food Protection, 70, 667–673. 10.4315/0362-028x-70.3.667 [DOI] [PubMed] [Google Scholar]
- Kästli P and Moosbrugger GA, 1968. Destruction of the foot‐and‐mouth disease virus in milk products through temperature. Schweizer Archiv fur Tierheilkunde, 110, 89–94. [PubMed] [Google Scholar]
- Kononov A, Prutnikov P, Shumilova I, Kononova S, Nesterov A, Byadovskaya O, Pestova Y, Diev V and Sprygin A, 2019. Determination of lumpy skin disease virus in bovine meat and offal products following experimental infection. Transboundary and Emerging Diseases, 66, 1332–1340. 10.1111/tbed.13158 [DOI] [PubMed] [Google Scholar]
- Masana MO, Fondevila NA, Gallinger MM, Lasta JA, Rodriguez HR and Gonzalez B, 1995. Effect of low‐temperature long‐time thermal processing of beef‐cuts on the survival of foot‐and‐mouth disease virus. Journal of Food Protection, 58, 165–169. 10.4315/0362-028X-58.2.165 [DOI] [PubMed] [Google Scholar]
- McVicar JW, Singh EL, Mebus CA and Hare WC, 1986. Embryo transfer as a means of controlling the transmission of viral infections. VIII. Failure to detect foot‐and‐mouth disease viral infectivity associated with embryos collected from infected donor cattle. Theriogenology, 26, 595–603. 10.1016/0093-691x(86)90166-4 [DOI] [PubMed] [Google Scholar]
- Mebus CA, 1987. Report to the Infectious Diseases of Cattle Committee. In: Proceedings 91st Annual Meeting USAHA, United States Animal Health Association, Louisville, KY, October 25–30, 10. [Google Scholar]
- Mebus CA and Singh EL, 1991. Embryo transfer as a means of controlling the transmission of viral infections XIII. Failure to transmit foot‐and‐mouth disease virus through the transfer of embryos from viremic donors. Theriogenology, 35, 435–441. 10.1016/0093-691X(91)90293-M [DOI] [PubMed] [Google Scholar]
- Mebus C, Arias M, Pineda JM, Tapiador J, House C and Sánchez‐Vizcaíno JM, 1997. Survival of several porcine viruses in different Spanish dry‐cured meat products. Food Chemistry, 59, 555–559. 10.1016/S0308-8146(97)00006-X [DOI] [Google Scholar]
- Meegan JM, 1979. The Rift Valley fever epizootic in Egypt 1977–1978 1. Description of the epizootic and virological studies. Transactions of the Royal Society of Tropical Medicine and Hygiene, 73, 618–623. 10.1016/0035-9203(79)90004-x [DOI] [PubMed] [Google Scholar]
- Pantin‐Jackwood MJ and Swayne DE, 2009. Pathogenesis and pathobiology of avian influenza virus infection in birds. Revue Scientifique et Technique, 28, 113–136. 10.20506/rst.28.1.1869 [DOI] [PubMed] [Google Scholar]
- Rapoport E and Shimshony A, 1997. Health hazards to the small ruminant population of the Middle East posed by the trade of sheep and goat meat. Revue Scientifique et Technique, 16, 57–64. 10.20506/rst.16.1.993 [DOI] [PubMed] [Google Scholar]
- Salwa A and Gaber AS, 2007. Inactivation of foot and mouth disease virus in milk and milk products. Milchwissenschaft, 62, 1. [Google Scholar]
- Sellers RF, 1969. Inactivation of foot‐and‐mouth disease virus in milk. British Veterinary Journal, 125, 163–168. 10.1016/s0007-1935(17)49008-7 [DOI] [PubMed] [Google Scholar]
- Sonder E, Ackermann M, McCullough KC and Kihm U, 1990. Inactivation of foot and mouth disease virus in skimmed milk with propionic acid, citric acid and hydrogen peroxide. Revue Scientifique et Technique, 9, 1139–1155. 10.20506/rst.9.4.528 [DOI] [PubMed] [Google Scholar]
- Stringfellow DA and Givens MD, 2000. Epidemiologic concerns relative to in vivo and in vitro production of livestock embryos. Animal Reproduction Science, 60, 629–642. 10.1016/S0378-4320(00)00104-4 [DOI] [PubMed] [Google Scholar]
- Swayne DE and Beck JR, 2004. Heat inactivation of avian influenza and Newcastle disease viruses in egg products. Avian Pathology, 33, 512–518. 10.1080/03079450400003692 [DOI] [PubMed] [Google Scholar]
- Tomasula PM, Kozempel MF, Konstance RP, Gregg D, Boettcher S, Baxt B and Rodriguez LL, 2007. Thermal inactivation of foot‐and‐mouth disease virus in milk using high‐temperature, short‐time pasteurization. Journal of Dairy Science, 90, 3202–3211. 10.3168/jds.2006-525 [DOI] [PubMed] [Google Scholar]
- Walker JL, de Leeuw PW, Callis JJ and van Bekkum JG, 1984. The thermal death time curve for foot‐and‐mouth disease virus contained in primarily infected milk. Journal of Biological Standardization, 12, 185–189. 10.1016/S0092-1157(84)80052-9 [DOI] [PubMed] [Google Scholar]
- Wieringa‐Jelsma T, Wijnker JJ, Zijlstra‐Willems EM, Dekker A, Stockhofe‐Zurwieden N, Maas R and Wisselink HJ, 2011. Virus inactivation by salt (NaCl) and phosphate supplemented salt in a 3D collagen matrix model for natural sausage casings. International Journal of Food Microbiology, 148, 128–134. 10.1016/j.ijfoodmicro.2011.05.010 [DOI] [PubMed] [Google Scholar]
- WOAH (World Organisation for Animal Health) , 2021. Terrestrial Animal Health Code. Available online: https://www.WOAH.int/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Extensive literature search on the presence of selected Category A disease pathogens in germinal products
Extensive literature search on the presence of selected Category A disease pathogens in animal products, animal by‐products and feed of plant origin and straw
Extensive literature search on the effectiveness of risk mitigation treatments for products of animal origin and products of non‐animal origin


