Table 1. Phosphoramidites of Base-Modified Nucleosides Synthesized in this Work.
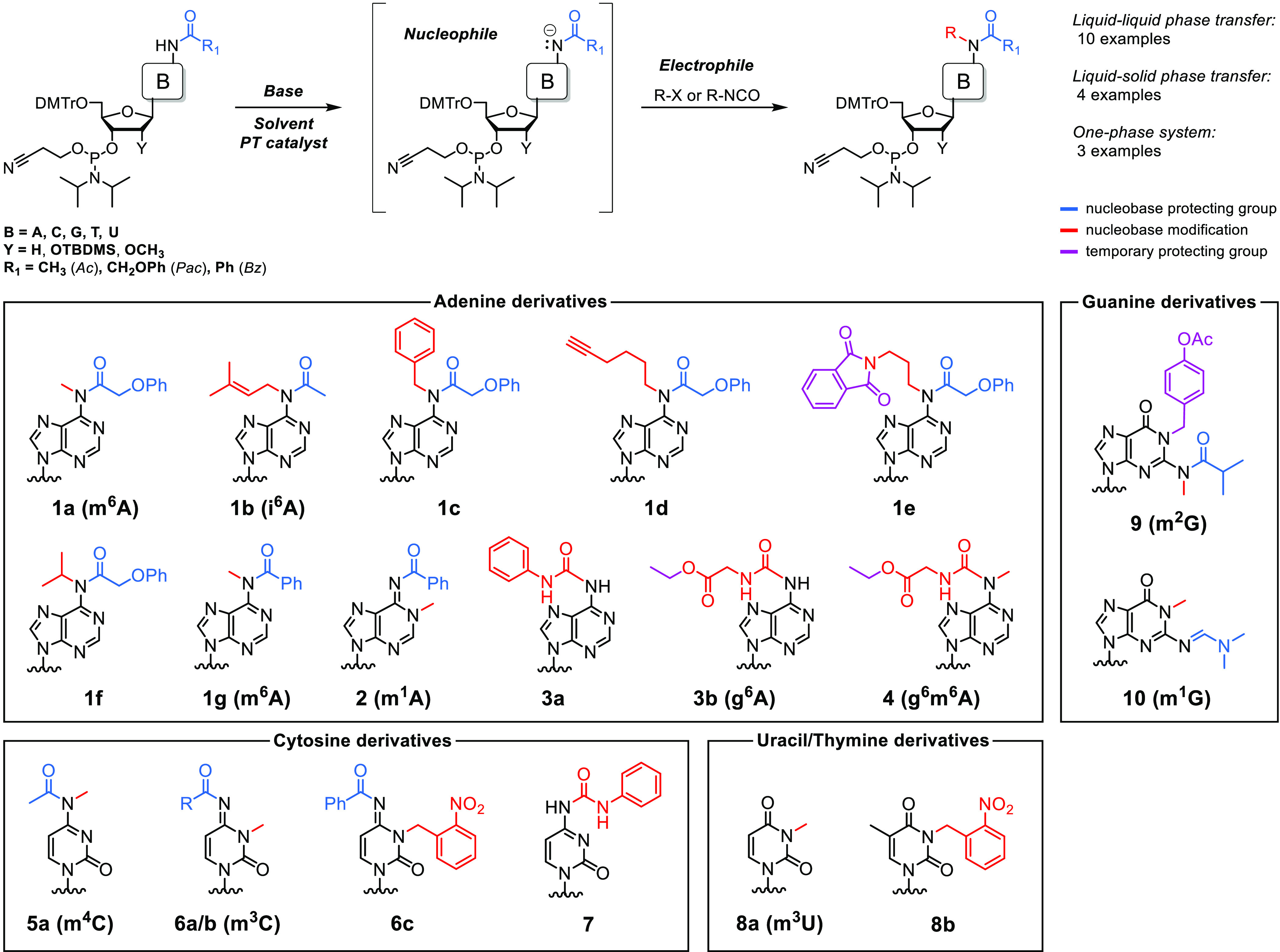
| product | nucleophilea | electrophile | base | solvent(s) | phase-transfer catalyst | yieldb |
|---|---|---|---|---|---|---|
| 1a | AmPac | methyl iodide | 1 M NaOH (aq) | CH2Cl2/H2O | Bu4NBr | 79% |
| 1b | AAc | isopentenyl bromide | 1 M NaOH (aq) | CH2Cl2/H2O | Bu4NBr | 80% |
| 1c | AmPac | benzyl bromide | 1 M NaOH (aq) | CH2Cl2/H2O | Bu4NBr | 59% |
| 1d | AmPac | 6-iodohex-1-yne | 1 M NaOH (aq) | CH2Cl2/H2O | Bu4NBr | 56% |
| 1e | AmPac | 3-phthalimidopropyl bromide | KOH/K2CO3 (s) | toluene | Bu4NBr | 48% |
| 1f | AmPac | 2-iodopropane | KOH/K2CO3 (s) | toluene | Bu4NBr | 45% |
| 1g + 2 | ABz | methyl iodide | KOH/K2CO3 (s) | toluene | Bu4NBr | 62% + 29% |
| 3a | AAc | phenyl isocyanate | triethylamine | CH2Cl2 | 57% | |
| 3b | AAc | ethyl isocyanatoacetate | triethylamine | CH2Cl2 | 83% | |
| 4 | g6A (3b) | methyl iodide | 1 M NaOH (aq) | CH2Cl2/H2O | Bu4NBr | 70% |
| 5a + 6a | CAc | methyl iodide | 1 M NaOH (aq) | CH2Cl2/H2O | Bu4NBr | 43% + 25% |
| 6b | CBz | methyl iodide | 1 M NaOH (aq) | CH2Cl2/H2O | Bu4NBr | 75% |
| 6c | CBz | 2-nitrobenzyl chloride | KOH/K2CO3 (s) | toluene | Bu4NBr | 73% |
| 7 | CAc | phenyl isocyanate | triethylamine | CH2Cl2 | 42% | |
| 8a | Um | methyl iodide | 1 M NaOH (aq) | CH2Cl2/H2O | Bu4NBr | 89% |
| 8b | T | 2-nitrobenzyl chloride | KOH/K2CO3 (s) | toluene | Bu4NBr | 71% |
| 9 | GiBu | 4-(iodomethyl)phenyl acetate, methyl iodide | 1 M NaOH (aq) | CH2Cl2/H2O | Bu4NBr | 12% |
| 10 | Gdmf | methyl iodide | 1 M NaOH (aq) | CH2Cl2/H2O | Bu4NBr | 82% |
The protecting group of the exocyclic amine in the nucleoside phosphoramidite is indicated by the superscript, as defined by R1 in the abovementioned reaction scheme; the 2′-C substituent (Y in the abovementioned reaction scheme) is −H for DNA amidites, tert-butyldimethylsilyloxyl (−OTBDMS) for RNA amidites, and −OCH3 for 2′-O-methylRNA amidites (denoted by a subscript “m).”
Isolated yield (flash chromatography).
