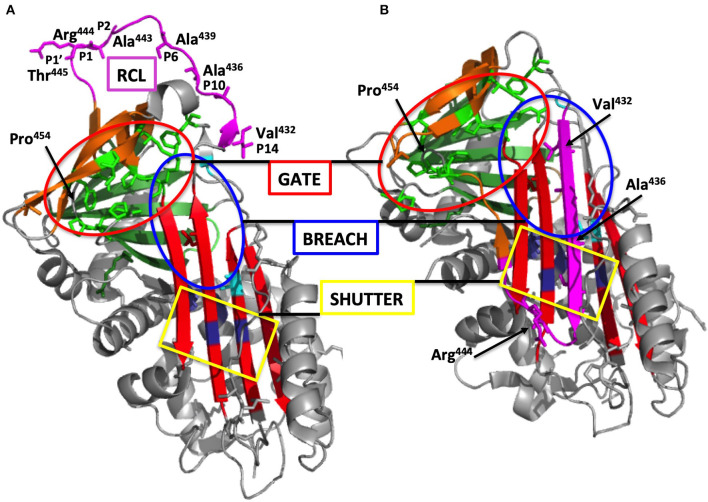
Figure 3.
Overall structure of C1-INH. (A) Native C1-INH serpin domain, with positions of strategic residues shown with side chains in sticks. Five regions for the serpin function are presented on the 3D-model of C1Inh (PDB ID 5DU3) presented using Pymol. The model starts at Phe100 and lacks a great part of the N-terminal domain (residues 1-112). Strategic functional regions are indicated with (i) the reactive site loop (RCL), colored purple, including Arg444 P1 and the hinge region (P15-P9), essential for protease recognition, RCL mobility and conformational transformation for its insertion as strand 4A (s4A) (i.e., S to R transition, after protease inhibition); (ii) the central β-sheet A, colored red, with the breach region, indicated by a blue ellipse, located at its top and point of initial insertion of RCL, and the shutter domain, with a yellow rectangle, close to the center of β-sheet A, that, with breach, assists sheet opening and accepts the insertion of conserved proximal hinge s4A between s3A and s5A; (iii) the gate, highlighted with green sticks and targeted with a red ellipse, including s3C and s4C of β-sheet C. β-sheets B and C are colored green and brown, respectively. (B) Latent form of C1-INH. The same regions involved in the serpin function but in a nonfunctional conformation where RCL is kept inside the central β-sheet A structure with additional s4A colored purple and remaining inaccessible to target proteases (PDB ID 20AY). Residue numbering was done according to mature C1-INH protein. Pictures were drawn by Dr. Christine Gaboriaud, Institut de Biologie Structurale, Grenoble France.