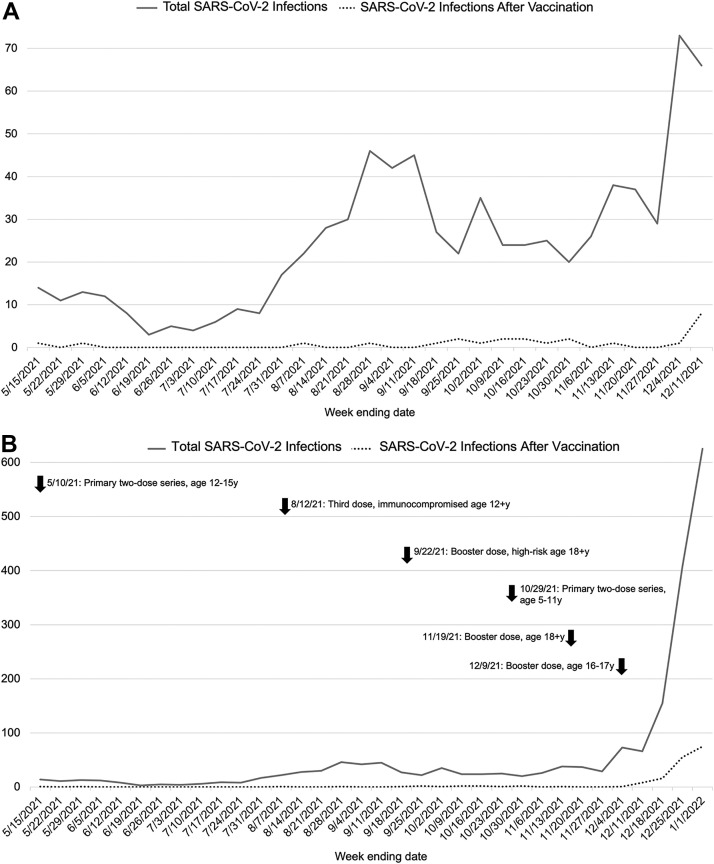
Figure 1.
Incidence of total SARS-CoV-2 infection and SARS-CoV-2 infection after vaccination at Lurie Children's during the A, pre-Omicron study period (May 15, 2021, to December 11, 2021, prior to the substantial surge in SARS-CoV-2 infection activity) and B, the complete study period (May 15, 2021, to January 1, 2022). The solid line represents the total incidence of pediatric SARS-CoV-2 infection, and the dashed line represents the total incidence of SARS-CoV-2 infection after vaccination. In B, arrows indicate date of Food and Drug Administration Emergency Use Authorization of Pfizer-BioNTech SARS-CoV-2 mRNA vaccine in children and young adults aged 0-20 years. Not shown is the initial authorization of SARS-CoV-2 vaccine in adolescents aged 16+ years on December 11, 2020.