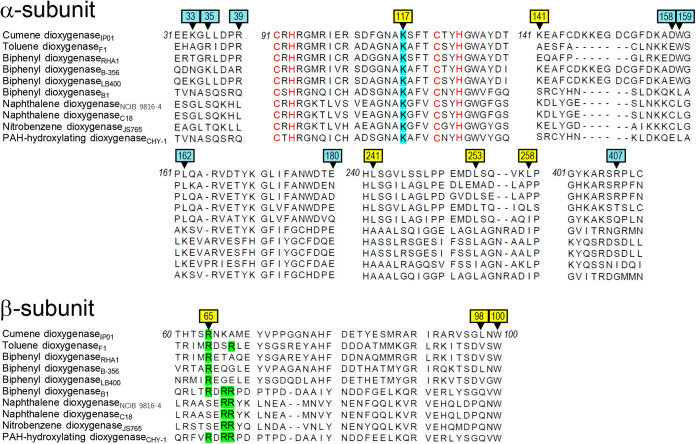
FIG 5.
Amino acid sequence alignments of α- and β-subunits of 10 structure-solved Oxys. Parts of amino acid sequence alignments of Oxy components of 10 RO systems are shown. Enzyme names followed by origins (subscripted) are shown at left. Alanine-substituted residues (arrowheads) at the top-wise and side-wise sides are shown by light blue and yellow backgrounds, respectively. Conserved positive amino acid residues in the side-wise putative Fd-binding site (Fig. 4) are shown in light blue and light green in the α- and β-subunit sequences, respectively. Cys and His residues in the α-subunit sequences involved in the coordination of Rieske clusters are shown in red. Numbers at termini show the positions of terminal amino acid residues in CumDO-O protein.