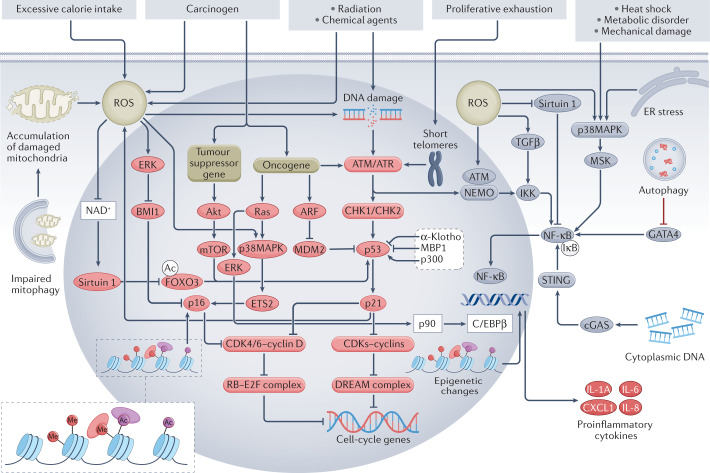
Fig. 1. Mechanisms of cellular senescence and the SASP.
DNA damage secondary to radiation, chemical agents or accumulation of reactive oxygen species (ROS) is the main cause of cellular senescence-inducing stress. Proliferation-induced telomere shortening can also activate the DNA damage response, which in turn leads to activation of the p53–p21 pathway, inhibition of cyclin-dependent kinase (CDK)–cyclin complexes and formation of the DREAM complex, which represses cell-cycle genes, leading to cell-cycle arrest and senescence. Activated p21 can also induce further ROS production, forming a vicious circle. In addition, impaired mitophagy can lead to mitochondrial dysfunction and excessive ROS production. ROS can induce senescence independently of the DNA damage response by activating the p16–RB pathway via activation of ERK, which inhibits BMI1 and thereby enables activation of p16, and by activating p38MAPK signalling, which upregulates ETS2 and in turn activates p16, and by inhibiting NAD+, which leads to reduced expression of sirtuin 1 and activation of FOXO3, which activates p53. P16 inhibits the formation of CDK4/6–cyclin D complexes and thereby promotes formation of the RB–2F complex, which inhibits the transcription of cell-cycle genes. Oncogene activation can activate the p53–p21 pathway not only via the DNA damage response, but also via the ARF–MDM2 signalling. In addition, oncogene activation can activate the p16–RB pathway via p38MAPK signalling. Loss of tumour suppressor genes induces senescence via Akt–mTOR signalling, which activates p53. Other factors that regulate the p53–p21 pathway include α-Klotho, MBP1 and p300. Epigenetic alterations such as methylation (Me) and acetylation (Ac) can induce senescence through the p16–RB pathway. NF-κB signalling regulates the senescence-associated secretory phenotype (SASP) and together with the transcription factor C/EBPβ, co-activates promoters of SASP genes, such as those that encode pro-inflammatory cytokines. The DNA damage response protein ATM together with NEMO activates the NF-κB–C/EBPβ signalling pathway. ROS can activate the SASP not only by promoting nuclear translocation of NEMO and activation of ATM, but also by inhibiting sirtuin 1 and activating p38MAPK and TGFβ, which in turn activate NF-κB. Heat shock, metabolic disorders, mechanical damage and endoplasmic reticulum (ER) stress can also activate the NF-κB–C/EBPβ pathway via p38MAPK signalling. Cytoplasmic DNA accumulation can trigger aberrant activation of cGAS-STING cytoplasmic DNA sensors and promote the SASP via activation of NF-κB. Impairment of autophagy hampers degradation of the transcription factor GATA4, which activates NF-κB and leads to initiation of the SASP. Notably, oncogenic Ras can activate C/EBPβ via ERK–p90 signalling and histone epigenetic changes can regulate the SASP independently of the NF-κB–C/EBPβ pathway. ARF, ADP-ribosylation factor; BMI1, B lymphoma Mo-MLV insertion region 1 homologue; cGAS, cyclic GMP–AMP synthase; CHK, checkpoint kinase; ERK, extracellular regulated protein kinases; ETS2, E26 transformation-specific proto-oncogene 2; E2F, early 2 factor; FOXO3, forkhead box protein O3; IKK, IκB kinase; MDM, mouse double minute 2; MSK, mitogen- and stress-activated protein kinase; NEMO, NF-κB essential modulator; STING, stimulator of interferon genes.